Novel process for synthesizing 3-deacetyl cefuroxime sodium (DCCF)
A technology of acetyl cefuroxime and acetyl cephalosporanic acid, which is applied in the new process field of 3-deacetyl cefuroxime acid synthesis, can solve the problems of solvent recovery difficulties, long production cycle, and many solvents, and achieve the reduction of solvent types, The effect of shortening the production cycle and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
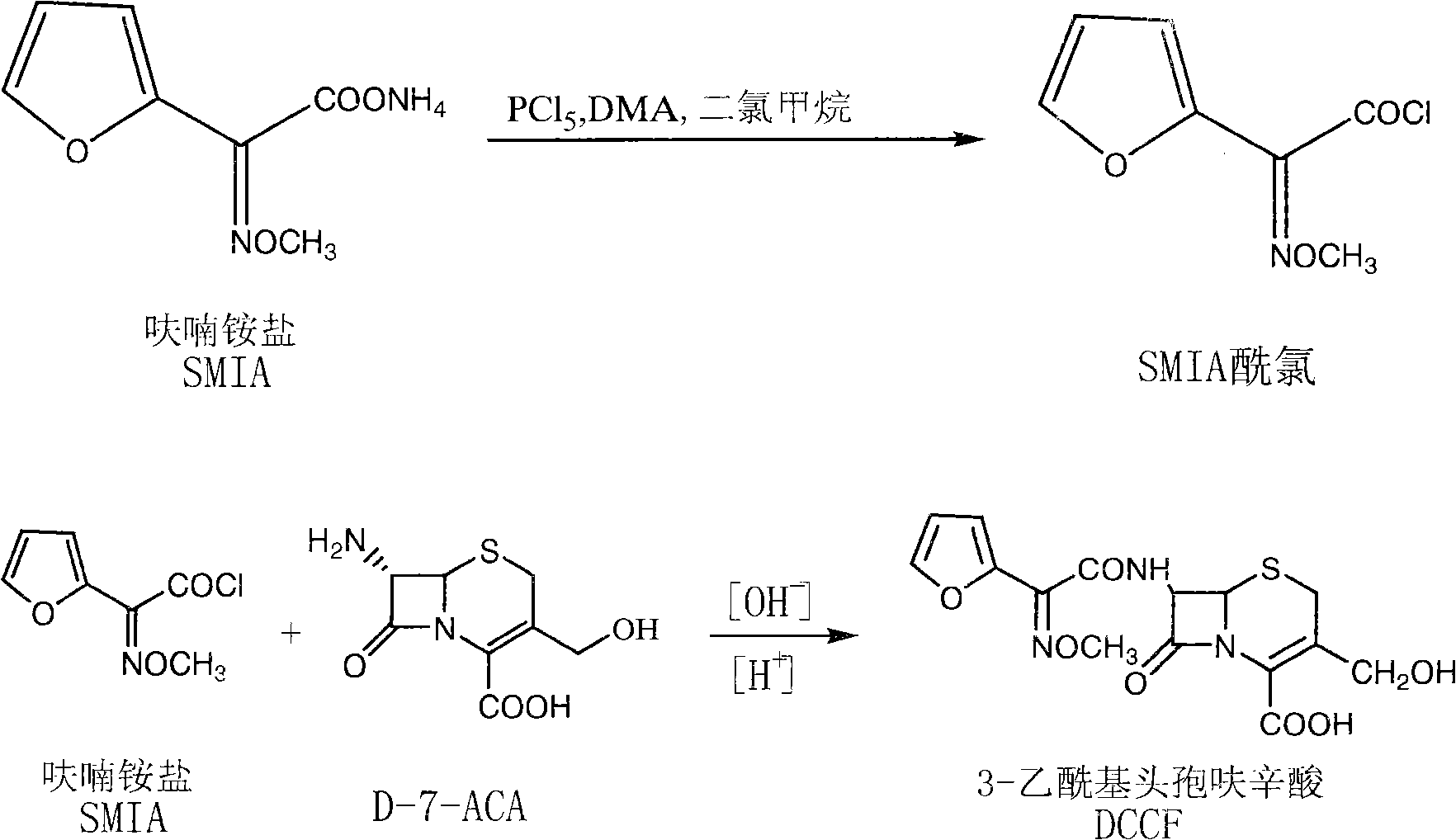
[0032] (1) Synthesis of cefuroxime side chain (SMFA acid chloride): add 4.16kg of phosphorus pentachloride (with D-7-ACA molar ratio range: 1:1) and 70L of dichloromethane into the cleaned and dried reaction tank and stir to dissolve , lower the temperature to: -15~-50°C, add 5L DMF continuously, control the feeding process for 10-15 minutes, and control the temperature not higher than -20°C, after the addition is completed, continue to add 3.72kg furan ammonium salt (with D-7- ACA molar ratio range: 1:1), control the temperature at -5°C to -25°C and stir for 90 minutes, wash with ice water three times after the reaction, and cool the organic phase to 15°C to -20°C for storage.
[0033] (2) Preparation of deacetylcefturoxime acid: Add 26L of purified water and 4.65kg of D-7-ACA to the cleaned reaction tank, cool down to 0-10°C, add NaOH solution to adjust the pH to 7.5-8.5 to dissolve, after the dissolution is complete , add the organic phase containing SMIA acid chloride prep...
example 2
[0035] (1) Synthesis of cefuroxime side chain (SMFA acid chloride): add 8.4kg phosphorus pentachloride (with D-7-ACA molar ratio: 2:1) and 70L dichloromethane into the cleaned and dried reaction tank and stir to dissolve , lower the temperature to: -15~-50°C, add 5L DMA continuously, control the feeding process for 10-15 minutes, control the temperature not higher than -20°C, after the addition is complete, continue to add 7.45kg furan ammonium salt (with D-7- The molar ratio of ACA is 2:1), and the temperature is controlled at -5°C to -25°C to stir and react for 90 minutes. After the reaction is completed, wash with ice water three times, and the organic phase is cooled to 15°C to -20°C for storage.
[0036] (2) Preparation of deacetylcefturoxime acid: Add 26L of purified water and 4.65kg of D-7-ACA to the cleaned reaction tank, cool down to 0-10°C, add NaOH solution to adjust the pH to 7.5-8.5 to dissolve, after the dissolution is complete , add the organic phase containing ...
example 3
[0038] (1) Synthesis of cefuroxime side chain (SMFA acid chloride): add 20.84kg of phosphorus pentachloride (with D-7-ACA molar ratio: 5:1) and 150L of dichloromethane into the cleaned and dried reaction tank and stir to dissolve , lower the temperature to: -15~-50°C, add 10L DMF continuously, control the feeding process for 10-15 minutes, control the temperature not higher than -20°C, after the addition, continue to add 18.6kg furan ammonium salt (with D-7- The molar ratio of ACA is 5:1), the temperature is controlled at -5°C to -25°C and the reaction is stirred for 90 minutes, after the reaction is completed, it is washed with ice water three times, and the organic phase is cooled to 15°C to -20°C and stored for later use.
[0039] (2) Preparation of deacetylcefturoxime acid: add 26L of purified water and 4.65kg of D-7-ACA to the cleaned reaction tank, cool down to 0-10°C, add NaOH solution to adjust the pH to 7.5-8.5 to dissolve, after the dissolution is complete , add the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com