Composite nasal cavity preparations for curing allergic rhinitis
A technology for allergic rhinitis and preparations, applied in the field of medicine, can solve the problems of long cycle, poor treatment effect, and large side effects of drugs, etc., and achieve the effect of safe preparations, rapid action, and symptom improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
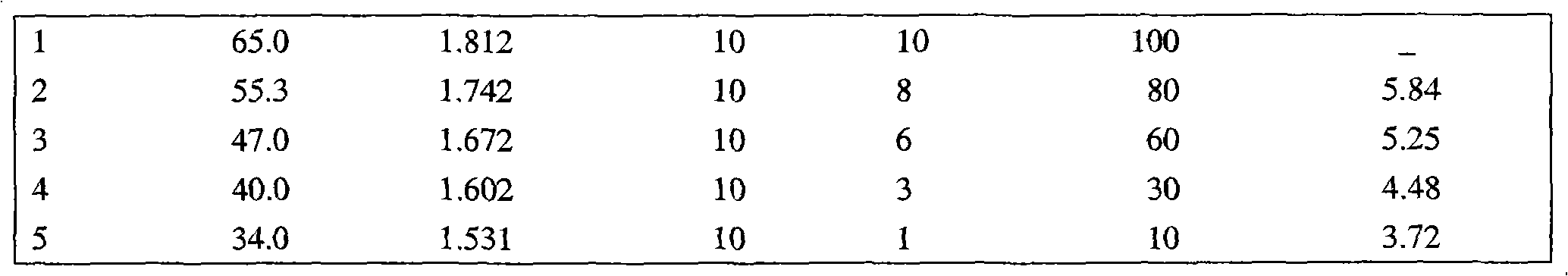
[0037] According to the following formula, nasal drops are prepared by adding auxiliary materials according to conventional pharmaceutical methods.
[0039] Chlorpheniramine: 41%;
[0040] Dexamethasone acetate: 16%;
[0041] Warin: 8%;
[0042] Accessories: balance
Embodiment 2
[0044] According to the following formula, nasal spray is prepared by adding auxiliary materials according to conventional pharmaceutical methods.
[0045] Anisodamine: 7%;
[0046] Diphenhydramine: 35%;
[0047] Hydrocortisone Sodium Phosphate: 22%;
[0048] P-light ephedrine: 14%;
[0049] Accessories: balance
Embodiment 3
[0051] According to the following formula, the ointment is prepared by adding auxiliary materials according to conventional pharmaceutical methods.
[0052] Anisodamine: 4%;
[0053] Chlorpheniramine: 38%;
[0054] Dexamethasone acetate: 19%;
[0055] Warin: 11%;
[0056] Accessories: balance
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com