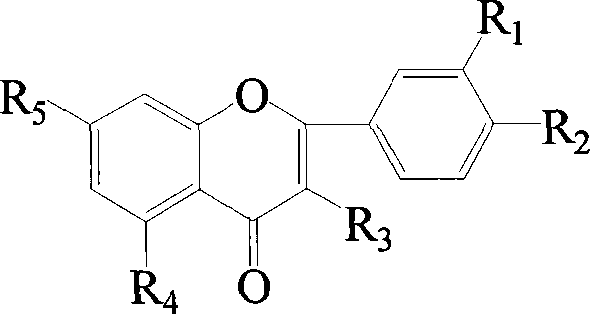
Flavonol sulfonates derivatives and method for synthesizing same
A technology of flavonol sulfonate and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of low bioavailability, poor absorption, slow effect and the like, and achieve the effects of high bioavailability, improved solubility and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1 compound 6
[0046] 7-Benzenesulfonyloxy-3,5,3',4'-tetrabenzylquercetin
[0047] Synthesis of intermediate 7-hydroxy-3,5,3',4'-tetrabenzylquercetin 11,12. Take quercetin (0.4mmol, 0.1208g) and disperse it in 10mL of dichloromethane, add 0.2024g of benzyl chloride, and store at -10°C under the protection of Ar. Incubate for 0.5-24 hours. Filter the mixture, spin the filtrate to dryness under reduced pressure, and purify by column chromatography (chloroform / petroleum ether=10:1) to obtain pure tetrabenzylquercetin 11 and tribenzylquercetin 12. Intermediate 11: m.p.
[0048] 140-142°C (literature value: 140-142°C); intermediate 12: m.p.151-153°C (literature value: 150-152°C).
[0049] Synthesis of 7-benzenesulfonyloxy-3,5,3',4'-tetrabenzylquercetin 6. Take 11 (0.4mmol, 0.2648g) and disperse it in 10mL of dichloromethane, add 0.05g of benzenesulfonyl chloride, and store at -20°C under the protection of Ar. Incubate for 0.5-48 hours. Filter...
Embodiment 2
[0051] The synthesis of embodiment 2 compound 3
[0052] 7-Benzenesulfonyloxyquercetin
[0053] Synthesis of 7-Benzenesulfonyloxyquercetin 3. Take 6 (0.4mmol, 0.3208g) and add 10-20% reactant weight Pd / C (10%) and 60-90mL tetrahydrofuran-absolute ethanol (2:1-1:1), and replace the air with hydrogen repeatedly three times Then, stir with hydrogen at room temperature for 24-48 hours. The catalyst was removed by filtration, washed twice with THF, the solvent was distilled off under reduced pressure, and dried in vacuo to obtain a yellow solid.
[0054] 1 H NMR (400MHz, CDCl 3 ): δ6.23(J=1.8Hz, 1H, H6); 6.51(d, J=1.8Hz, 1H, H8), 7.06(d, J=8.8Hz, 1HH5'), 7.32(t, 1H, J =7.6Hz Phenylsulfonyl-4-H), 7.54(t, 2H, J=7.6Hz Phenylsulfonyl-3, 5-H), 7.87-7.95(m, 2H, H2', H6'), 8.11(d, 2H, J=7.8Hz, Phenylsulfonyl-2, 6-H), 9.56(s, 1H, OH), 10.41(s, 1H, OH), 10.74(brs, 1H, OH), 13.40(s, 1H, OH). m / z(EI)803.31(M + +1, 100%).
Embodiment 3
[0055] The synthesis of embodiment 3 compound 7
[0056] 4’,7-Diphenylsulfonyloxy-3,5,3’-tribenzylquercetin
[0057] Take 12 (0.4mmol, 0.2288g) and disperse it in 10mL of dichloromethane, add 0.05g of benzenesulfonyl chloride, and store at -20°C under the protection of Ar. Incubate for 0.5-48 hours. Filter the mixture, spin the filtrate to dryness under reduced pressure, and purify by column chromatography (chloroform / petroleum ether=20:1) to obtain a pure product.
[0058] 1 H NMR (400MHz, CDCl 3 ): δ4.99 (s, 2H, OCH 2 Ph), 5.12(s, 2H, OCH 2 Ph), 5.23(s, 2H, OCH 2 Ph), δ6.23(J=1.8Hz, 1H, H6); 6.51(d, J=1.8Hz, 1H, H8), 7.06(d, J=8.8Hz, 1H, H5'), 7.22-7.47( m, 17H, aromatic H), 7.54 (t, 4H, J = 7.6Hz Phenylsulfonyl-3, 5-H), 7.87-7.95 (m, 2H, H2', H6'), 8.11 (d, 4H, J = 7.8Hz, Phenylsulfonyl-2,6-H).m / z(EI)853.07(M + +1, 100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com