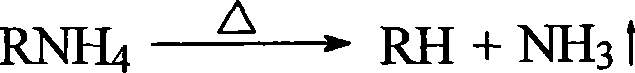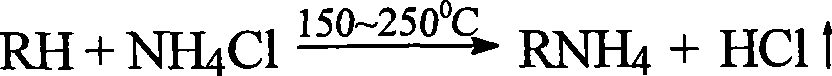Method for decomposing chloride and releasing hydrogen chloride by using non-volatile acid or acidic salt
A non-volatile acid and acid salt technology, which is applied in the direction of chlorine/hydrogen chloride and chloride preparation, etc., can solve the problems of high crystal size requirements for raw materials, low decomposition rate of ammonium chloride, and difficult heat transfer, etc., and achieve atomic The effect of high utilization rate, low price and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 1050 g of 85% phosphoric acid into a reactor equipped with a stirrer, a thermometer, and a reflux condenser, heat to 150°C, grind 70 g of ammonium chloride solid, and slowly put it into the reactor, heat up to 200°C, and pass it in Nitrogen, the nitrogen flow rate is 40L / h, the condenser is connected to the pipe, the hydrogen chloride gas is absorbed with distilled water, and the temperature is controlled to react for 2 hours. After the reaction, the yield of hydrogen chloride was 95.2%.
[0023] After the reaction, the resulting mixture was heated to 230° C., and the ammonia gas was absorbed and released with an aqueous hydrogen chloride solution. The reaction was carried out for 1.5 hours, and the ammonia yield was 90%.
[0024] The solid salt obtained after the above discharging ammonia is used in a repeated cycle, the first step is repeated, and the reaction is 2 hours, and the yield of hydrogen chloride is 96.2%.
Embodiment 2
[0026] Add 560g of solid sodium bisulfate into a reactor equipped with a stirrer, a thermometer and a reflux condenser, heat to 200°C, grind 140g of ammonium chloride solid, and slowly put it into the reactor, heat up to 250°C, and pass in Nitrogen, the nitrogen flow rate is 70L / h, the condenser is connected to the pipe, the hydrogen chloride gas is absorbed with distilled water, and the temperature is controlled for 1.5 hours. After the reaction, the yield of hydrogen chloride was 99%.
[0027] After the reaction, the resulting mixture was heated to 320°C, and ammonia gas was absorbed and released with an aqueous hydrogen chloride solution. The reaction was carried out for 1.5 hours, and the ammonia yield was 90%.
[0028] The solid salt obtained after the above discharging ammonia is used in a repeated cycle, the first step is repeated, and the reaction is carried out for 1.5 hours, and the yield of hydrogen chloride is 98.6%.
Embodiment 3
[0030] Add 1200g of 98% sulfuric acid into a reactor equipped with a stirrer, a thermometer and a reflux condenser, heat it to 160°C, grind 214g of ammonium chloride solid, and slowly put it into the reactor, heat up to 220°C, and pass it in Nitrogen, the nitrogen flow rate is 100L / h, the condenser is connected to the pipe, the hydrogen chloride gas is absorbed with distilled water, and the temperature is controlled to react for 1.8 hours. After the reaction, the measured yield of hydrogen chloride was 97.6%.
[0031] After the reaction, the resulting mixture was heated to 330°C, and ammonia gas was absorbed and released with an aqueous hydrogen chloride solution. The reaction was carried out for 1.5 hours, and the ammonia yield was 90%.
[0032] The solid salt obtained after the above discharging ammonia is used in a repeated cycle, the first step is repeated, and the reaction is performed for 1.8 hours, and the yield of hydrogen chloride is 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com