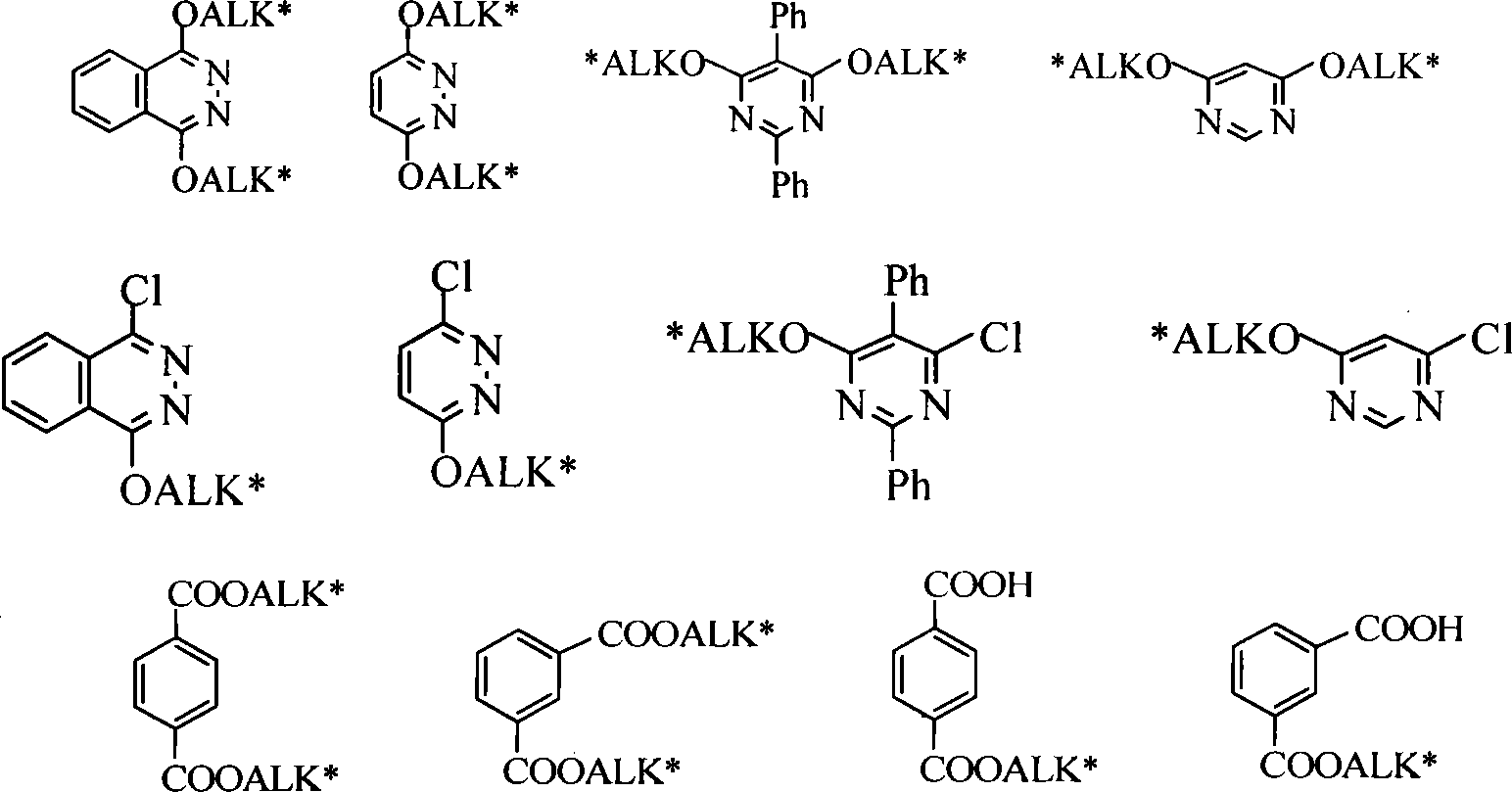
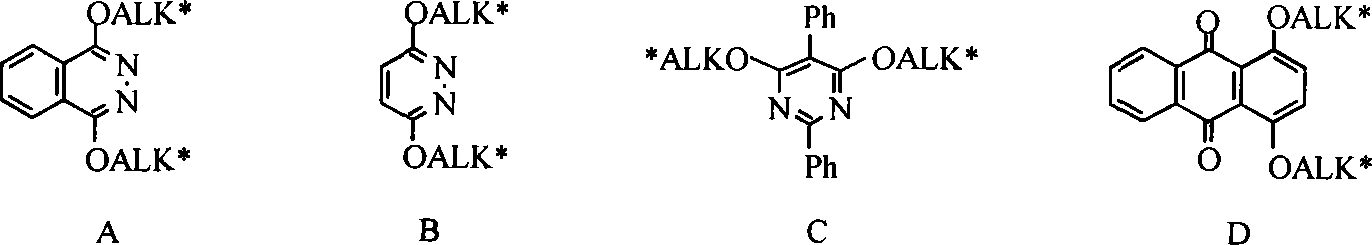
Method for synthesizing cinchona alkaloids microwave radiation non-solvent
A technology of cinchona alkaloids and a synthesis method is applied in the field of solvent-free synthesis of cinchona alkaloid derivatives by microwave irradiation, and the effects of increasing power, mild reaction conditions and prolonging reaction time are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of derivative 1:
[0027] In the 50mL round bottom flask, add 0.295g (0.0015mol) 1,4-dichloro-2,3 naphthyridine, 0.487g (0.0015mol) quinine, 0.84g (0.006mol) K 2 CO 3 , a catalytic amount of tetrabutylammonium bromide (TBAB), magnetically stirred and mixed evenly, placed in a microwave reactor, adjusted power 850W, temperature 125°C, reacted for 10min, monitored by TLC, added CH 2 Cl 2 After extraction (25 mL×3), the organic layers were combined, concentrated under reduced pressure, and separated by column chromatography to obtain 0.58 g of a light yellow solid with a yield of 68.7%.
Embodiment 2
[0028] Embodiment 2: the synthesis of derivative 2:
[0029] In the 50mL round bottom flask, add 0.295g (0.0015mol) 1,4-dichloro-2,3 naphthyridine, 0.97g (0.003mol) quinine, 1.7g (0.012mol) K 2 CO 3 , a catalytic amount of tetrabutylammonium chloride, magnetically stirred and mixed evenly, placed in a microwave reactor, adjusted power 850W, temperature 135°C, reacted for 5min, monitored by TLC, added CH after the reaction was completed 2 Cl 2 Extract (25mL×3), combine the organic layers, concentrate under reduced pressure, and separate by flash column chromatography, the yield is 73.8%.
Embodiment 3
[0030] Embodiment 3: the synthesis of derivative 3:
[0031] In the 50mL round bottom flask, add 1.77g (0.006mol) cinchonine, 1.19g (0.006mol) 1,4-dichloro-2,3 naphthyridine, 3.38g (0.024mol) K 2 CO 3 , a catalytic amount of tetrabutylammonium bromide (TBAB), magnetically stirred and mixed evenly, placed in a microwave reactor, adjusted power 800W, temperature 120°C, reacted for 10min, monitored by TLC, added CH 2 Cl 2 After extraction (75mL×3), the combined organic layers were concentrated under reduced pressure and separated by flash column chromatography with a yield of 75.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com