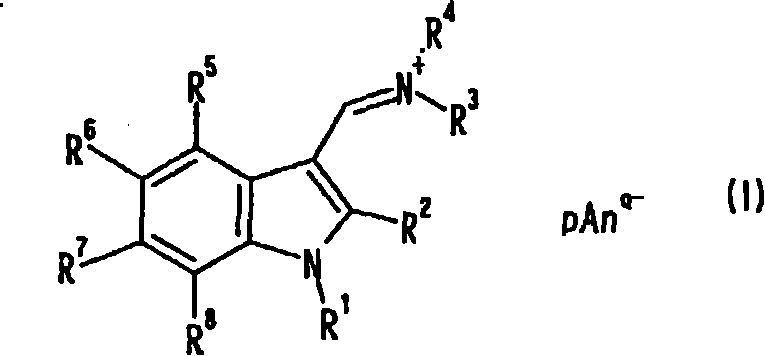
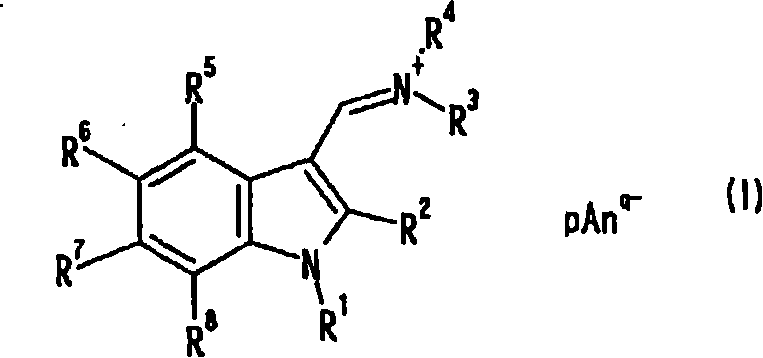
Indole compound, optical filter and optical recording material
A technology of indole compound and optical filter, which is applied in the direction of record carrier material, optical record carrier, recording/reproducing by optical method, etc., and can solve the problem that the absorption wavelength characteristics may not meet the requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] [Example 1] Synthesis of Compound No.1
[0079] 10 ml of dimethylformamide (DMF) and 80 ml of chloroform were placed in the reaction flask replaced with nitrogen, and 6.91 g (45.1 mmol) of phosphorus oxychloride was added dropwise under ice cooling, followed by stirring for 1 hour under ice cooling. A solution obtained by dissolving 3.94 g (30.0 mmol) of 1-methylindole in 40 ml of chloroform was added dropwise under ice-cooling, followed by heating under reflux for 3 hours. After cooling to room temperature, the reaction solution was slowly added dropwise to 23.1 g (126 mmol) of potassium hexafluorophosphate in 400 ml of an aqueous solution, and stirred at room temperature for 2 hours. The precipitate was filtered off, and washed with 200 ml of methanol while stirring at room temperature for 30 minutes. After filtering and drying, 9.32 g of pink solids were obtained (yield 93.4%). Confirmation: the obtained pink solid is the target substance, compound No.1. The analy...
Embodiment 2
[0088] [Example 2] Synthesis of Compound No.2
[0089] 10 ml of DMF and 80 ml of chloroform were placed in the reaction flask replaced with nitrogen, and 6.90 g (45.0 mmol) of phosphorus oxychloride was added dropwise under ice cooling, followed by stirring for 1 hour under ice cooling. A solution of 6.22 g (30.0 mmol) of 1-methyl-2-phenylindole dissolved in 40 ml of chloroform was added dropwise under ice-cooling, followed by heating under reflux for 3 hours. After cooling to room temperature, the reaction solution was slowly added dropwise to 400 ml of an aqueous solution of 20.0 g (109 mmol) of potassium hexafluorophosphate, and stirred at room temperature for 30 minutes. The water layer was removed, 400 ml of water was added, and the mixture was stirred at room temperature for 30 minutes. Then, the precipitate was filtered off, and washed with 150 ml of methanol while stirring at room temperature for 30 minutes. After filtering and drying, an off-white solid was obtained....
Embodiment 3
[0098] [Example 3] Synthesis of Compound No.3
[0099] 15 g (95.4 mmol) of N,N-di-n-butylformamide and 5.21 g (34.0 mmol) of phosphorus oxychloride were placed in the reaction flask after nitrogen substitution under ice cooling, and stirred for 1 hour under ice cooling. 2.62 g (20.0 mmol) of 1-methylindole was added under ice-cooling, and the mixture was stirred at 100° C. for 3 hours. After cooling to room temperature and distilling off N,N-di-n-butylformamide, 15.3 g (95.4 mmol) of potassium hexafluorophosphate was added and mixed uniformly. After adding 150 ml of water and stirring at room temperature for 30 minutes, the precipitate was filtered off, stirred with 100 ml of methanol at room temperature for 30 minutes, further added with 50 ml of water, and stirred at room temperature for 30 minutes to wash. After filtering and drying, a khaki solid was obtained. Recrystallized from acetonitrile / methanol mixed solvent, filtered and dried to obtain 2.90 g of light pink solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| visible light transmittance | aaaaa | aaaaa |
| hazing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com