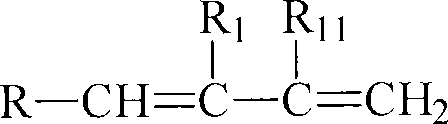Halobutyl elastomers
A kind of elastomer, butyl technology, applied in the field of halogenated butyl elastomer, can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0029] Material
[0030] 2,2,4,8,8-pentamethyl-4-nonene bromide (BPMN) was prepared as previously described (Parent, J.S. Thom, D.J. White, G. Whitney, R.A. Hopkins, W.J. Polym. Sci. Part A: Polym. Chem. 2001, 29, 2019-2026). The following reagents were used as received from Sigma-Aldrich (Oakville, Ontario): tetrabutylammonium hydroxide (1 M in methanol), tetrabutylammonium bromide (98%) (TBAB), trioctylmethyl chloride Ammonium chloride (Aliquat(R) 336, 95%), tert-butylacetic acid (98%), stearic acid (98%), benzoic acid (98%), 4-(dimethylamino)benzoic acid (98%), anthracene -9-Carboxylic acid (98%), linoleic acid (99%), and potassium hydroxide (99%). BIIR (Bayer BB2030) was used in the form supplied by LANXESS Corporation (Sarnia, Ontario). Tetrabutylammonium and potassium carboxylates are prepared by neutralization of the corresponding carboxylic acids with appropriate hydroxide bases.
[0031] equipment
[0032] The solid phase PTC reaction was performed using a Ha...
example 1
[0038] Example 1: (3,3-Dimethylbutyl)-2-(2,2-dimethylpropyl)prop-2-enyl 3,3-dimethylbutyrate (Example 1a) and ( Synthesis of 2E / Z)-6,6-dimethyl-2-(2,2-dimethylpropyl)hept-2-enyl 3,3-dimethylbutyrate (Example 1b-c) separate from
[0039] BPMN (0.022 g, 0.081 mmole), tetrabutylammonium tert-butylacetate (0.043 g, 0.120 mmole) and dodecane (0.4 ml) were sealed in a 1 ml Wheaton bottle and heated to 100° C. for one hour . The product was purified by column chromatography (silica gel, hexane) and isolated under vacuum to yield a yellow oil. Exocyclic methylene isomer Example 1a can be separated by column chromatography using silica gel and a mixed solvent (hexane:acetone:diethyl ether). FT-IR analysis: 1734cm -1 (C=O); MS analysis: C 20 h 38 o 2 Desired mass was 310.5m / e, found 311.4m / e [M+H] + (CI+) and 333.21m / e[M+Na] + (ESI+); 1 H NMR (CDCl 3 ) Example 1a: δ 5.18 (dd, 1H, -CHOC(O)-), 5.09 (s, 1.04H, =CH 2 ), 4, 86 (s, 1.06, =CH 2 ), 2.20 (dd, 2.1H, -CH 2 -), 1.92 (...
example 2
[0040] Example 2: (3,3-Dimethylbutyl)-2-(2,2-Dimethylpropyl)prop-2-enyl stearate (Example 2a) and (2E / Z)-6 , Synthesis and isolation of 6-dimethyl-2-(2,2-dimethylpropyl)hept-2-enyl stearate (Example 2b-c)
[0041] BPMN (0.044 g, 0.163 mmole), tetrabutylammonium stearate (0.085 g, 0.162 mmole) and dodecane (0.4 mL) were sealed in a 1 mL Wheaton bottle and heated to 100°C for one hour. The product was purified by column chromatography (silica gel, hexane) and isolated in vacuo to yield a yellow oil. FT-IR analysis: 1734cm -1 (C=O). MS Analysis: C 32 h 62 o 2 Desired mass is 478.83m / e, found 478.5m / e(EI+) and 479.5m / e[M+H] + (CI+). 1 H NMR (CDCl 3 ) Example 2: δ0.6-2.4 (m, 108, 2H, 3x-C (CH 3 ) 3 , 19x-CH 2 -), 4.4-5.6(m, 3.3H, 1x-CHOC(O)-, 2x=CH 2 , 2x-CH 2 OC(O)-, 2x=C-H); Example 2a: δ5.17(dd, -CHOC(O)-), 5.07(s,=CH 2 ), 4.84(s,=CH 2 ); Example 2b: δ4.49 (s, -CH 2 OC(O)-), 5.52(t,=C-H); Example 2c: δ4.56(s,-CH 2 OC(O)-), 5.39(t, =C-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com