Medicine for treating infectious diarrhea and peptic ulcer, preparing method and applications thereof
A technology for peptic ulcer and infectious diarrhea, applied in the digestive system, drug combination, medical formula, etc., can solve the problem of not neutralizing gastric acid, etc., achieve strong effect, do not cause renal insufficiency, and do not damage visual function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
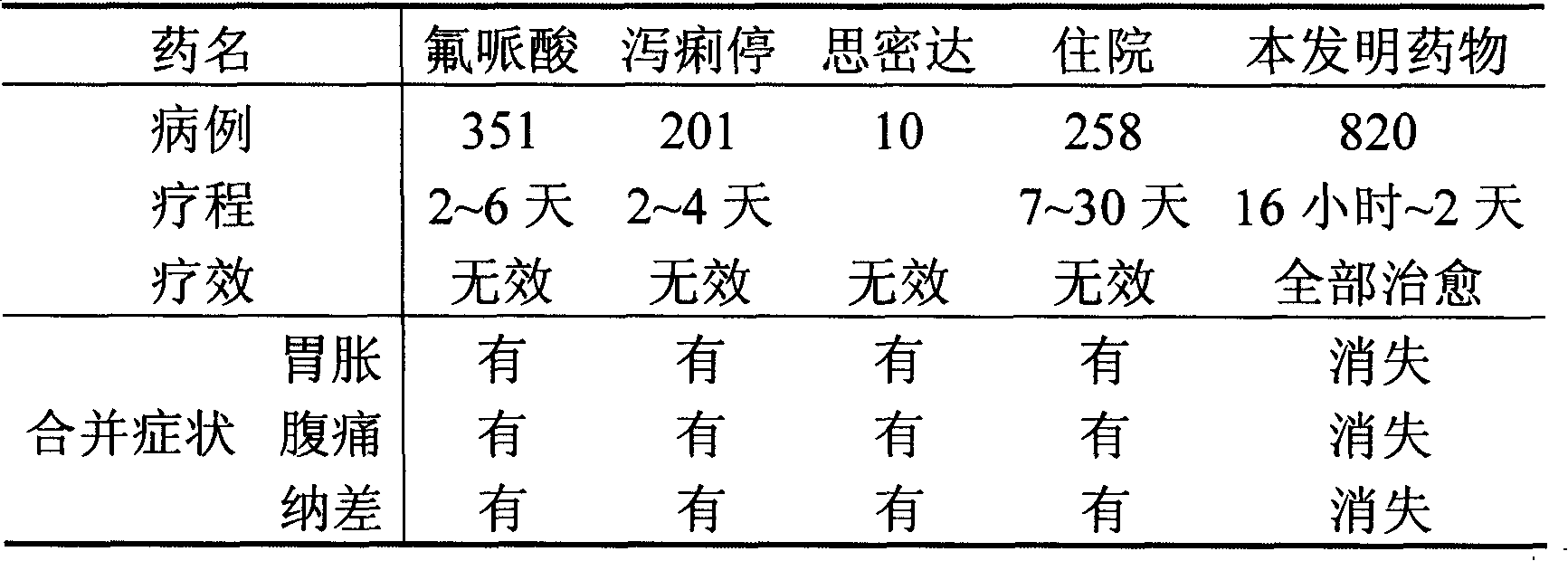
Image
Examples
Embodiment 1
[0091] Rifaximin 100g, bismuth subnitrate 300g, starch 20g, hypromellose 17g, magnesium stearate 2.5g.
[0092] According to the conventional preparation method, the above-mentioned raw materials are crushed and mixed uniformly, granulated with 3% hypromellose as a binder, dried, granulated, and encapsulated to obtain a capsule, which is made into 1000 capsules; it can also be compressed The tablets are made into tablets; or packed into granules; or the raw and auxiliary materials are directly mixed, bagged, and made into powders.
Embodiment 2
[0094] Rifaximin 50g, bismuth subnitrate 150g, starch 10g, hypromellose 17g, magnesium stearate 2.5g.
[0095] According to the conventional preparation method, the above-mentioned raw materials are pulverized and mixed uniformly, granulated with 3% hypromellose as a binder, dried, granulated, and encapsulated to obtain a capsule, which is made into 1000 capsules; it can also be compressed Tablets are made into tablets; or packaged into granules; or raw and auxiliary materials are directly mixed, bagged, and made into powders.
Embodiment 3
[0097] Rifaximin 100g, bismuth subcarbonate 600g, starch 10g, low-substituted hydroxypropyl cellulose 15g, hypromellose 17g, magnesium stearate 2.5g.
[0098] According to the conventional preparation method, the above-mentioned raw materials are pulverized and mixed uniformly, granulated with 3% hypromellose as a binder, dried, granulated, and encapsulated to obtain a capsule, which is made into 1000 capsules; it can also be compressed Tablets are made into tablets; or packaged into granules; or raw and auxiliary materials are directly mixed, bagged, and made into powders.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com