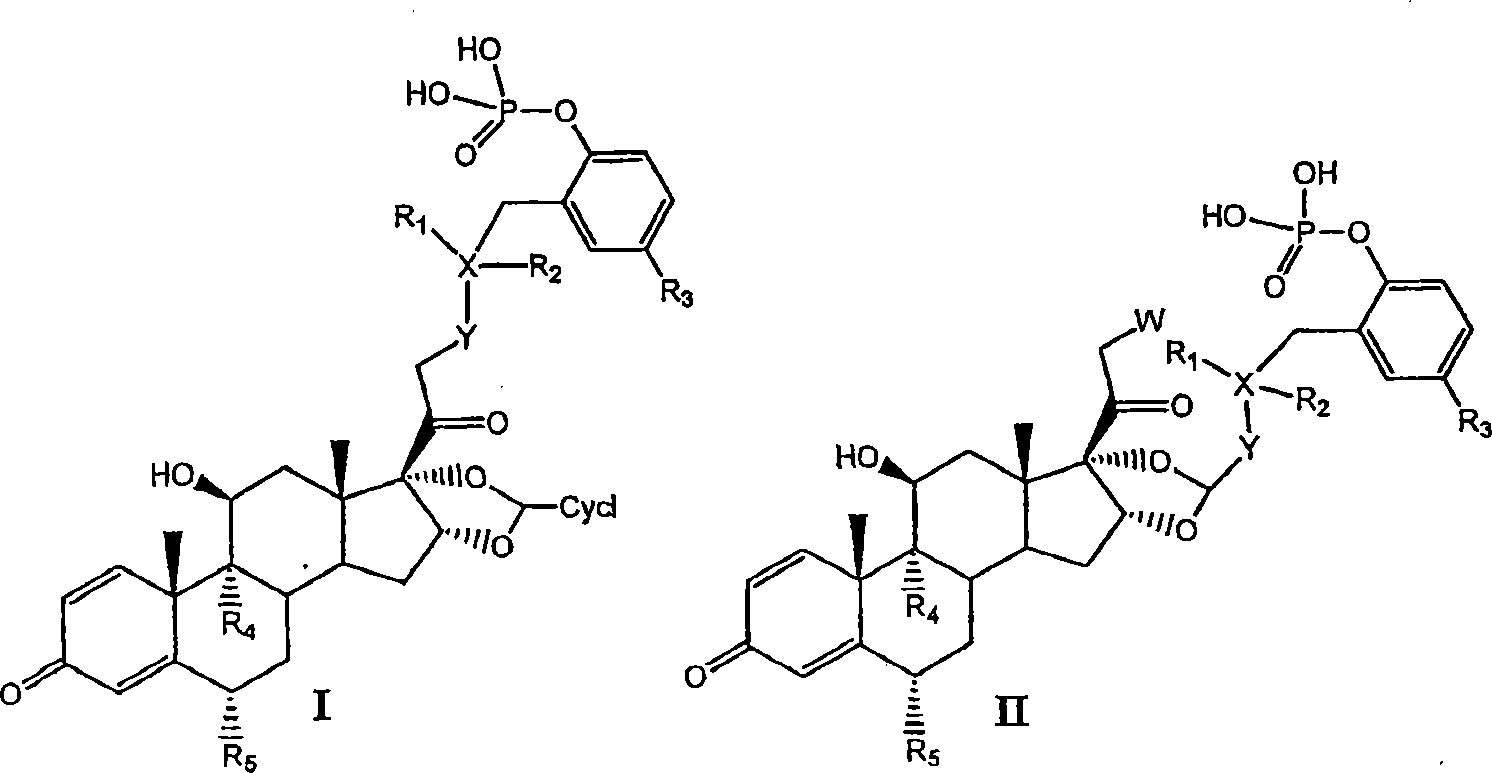
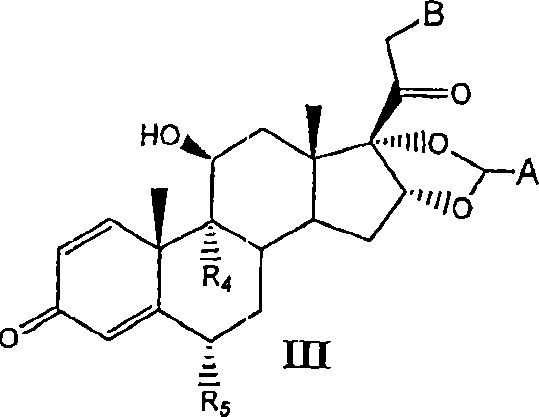
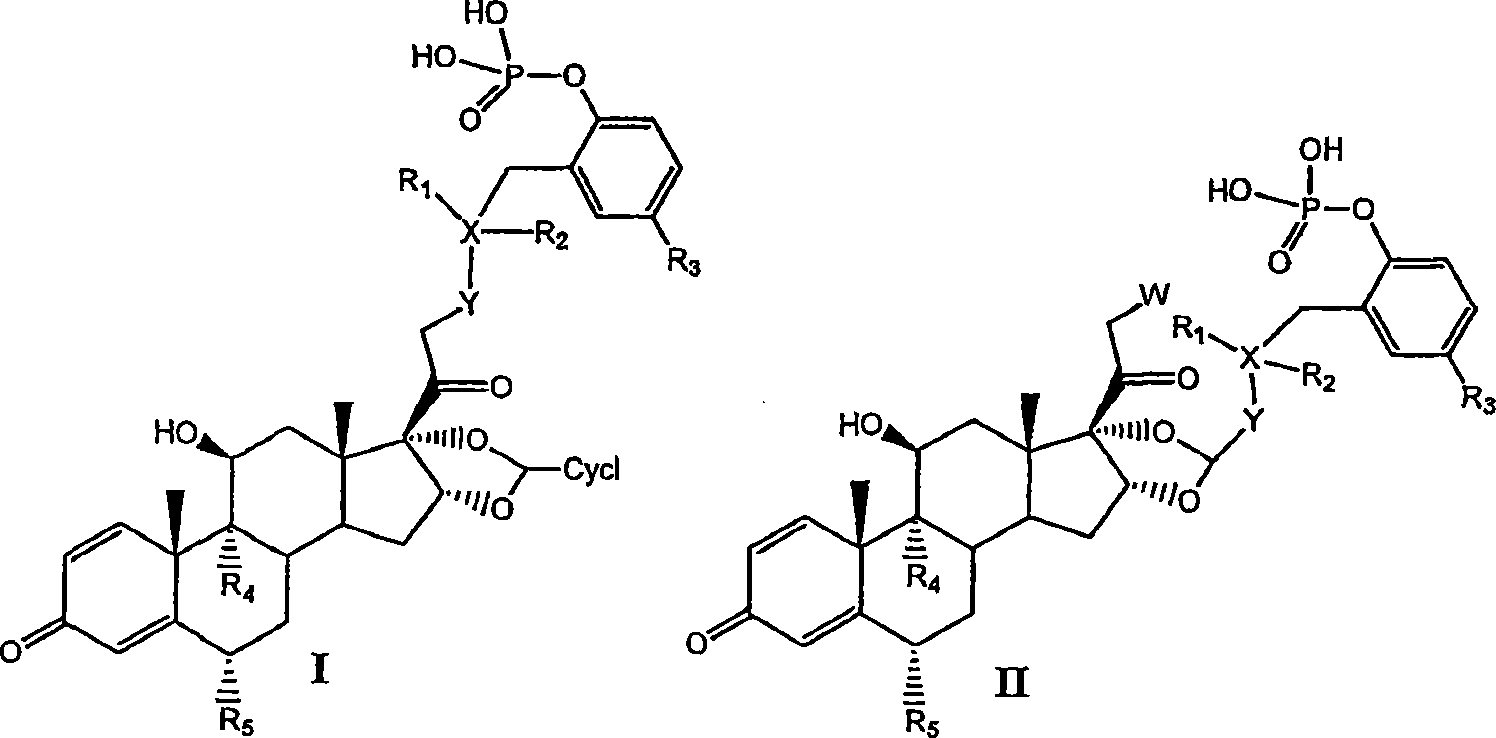
Substituted phenylphosphates as mutual prodrugs of steroids and beta-agonists for the treatment of pulmonary inflammation and bronchoconstriction
A phosphate ester, alkyl technology, applied in the field of new synergistic prodrugs, can solve the problem of not getting a completely satisfactory substitute for glucocorticoid therapy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0081] I. Preparation of Compounds of the Invention
[0082] Compounds of the invention can be prepared by the methods illustrated in Figures I-VII.
[0083] Concentrated routes to synergistic corticosteroid-β-agonist prodrugs include:
[0084] a) Synthesis of active phosphate-β-agonist derivatives (Figures I, II and III);
[0085] b) Preparation of steroid analogs (Figures IV and V);
[0086] c) Alkylation of steroid analogs with active β-agonist derivatives followed by final deprotection (Figures VI and VII).
[0087]
[0088] Figure II
[0089]
[0090] Figure III
[0091]
[0092] Figure IV
[0093]
[0094] Figure V
[0095]
[0096] Figure VI
[0097]
[0098] Figure VII
[0099]
[0100] For example, synergistic prodrug 17 (Example 133)
[0101] R 3 =(CH 2 ) 6 O(CH 2 ) 4 Ph
[0102] R 4 = F; R 5 = H;
[0103] Y-X(R 1 R 2 ) = 3-pyridinium
[0104] The synthesis of phosphate functionalized protected β-agonist derivatives is shown ...
Embodiment 1
[0127] Di-tert-butyl bromophosphate
[0128]
[0129] The title phosphorylation reagent was prepared following conditions modified from those described by Gajda and Zwierzak (1976). By lowering the reaction temperature to 15°C and reducing the reaction time to 2.5 hours, we obtained the title compound with better purity than when applying literature conditions (4 hours at 25°C). The title phosphorobromoester was unstable and was used immediately in the phosphorylation reaction (see Examples 4, 11 and 14).
Embodiment 2
[0132] [2-Hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethyl]-[6-(4-phenyl-butoxy)-hexyl-ammonia tert-butyl carbamate
[0133]
[0134] Commercially available salmeterol xinafoate (6.04 g, 10 mmol) and potassium carbonate (1.39 g, 10 mmol) were suspended in 1,4-dioxane / water mixture (1:1, 80 mL) with stirring middle. Then, di-tert-butyl dicarbonate (2.40 g, 11 mmol) dissolved in 1,4-dioxane (10 mL) was added dropwise while stirring was continued at room temperature. TLC analysis after 30 minutes showed only traces of starting material. After 2 hours, 1,4-dioxane was evaporated and the resulting suspension was diluted with water and extracted twice with chloroform (total 125 mL). Then, the organic layer was washed with saturated sodium bicarbonate, brine, and dried over anhydrous magnesium sulfate. The crude material obtained after decantation and evaporation is purified by chromatography on silica gel eluting with an ethyl acetate / hexane mixture (1:1). The title com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com