Method for producing cloned dog
A technology of nuclear transfer and embryos, applied in biochemical equipment and methods, botanical equipment and methods, special equipment for doors/windows, etc., can solve problems such as low cloning efficiency, difficulty in cloning dogs, and difficulty in practical application. Achieve the effects of improving cloning efficiency and optimizing electrofusion conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Extraction of dog recipient oocytes
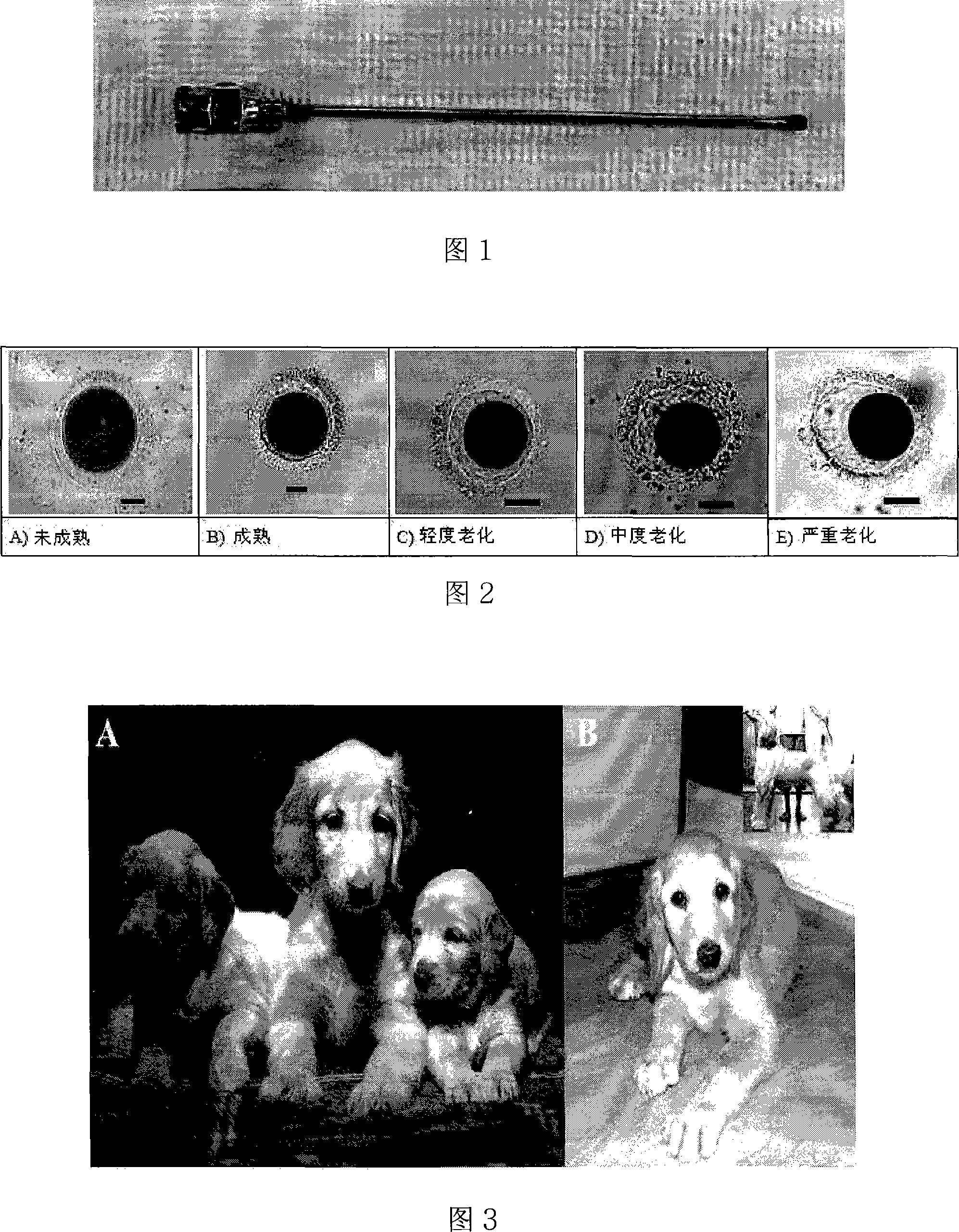
[0064] Twenty-three female mongrel dogs aged 1-5 years were used as oocyte donors. These dogs are cultivated in accordance with the standards approved and established by the Animal Culture Laboratory of Seoul National University. The ovulation period of the dog is determined by daily vaginal smear test and serum progesterone level analysis of dogs in natural estrus, and mature oocytes are obtained 48-72 hours after ovulation. The obtained oocytes include immature oocytes, mature oocytes, slightly aged oocytes, moderately aged oocytes and severely aged oocytes. The morphology of these oocytes is shown in Figure 2.
[0065] In order to measure the serum progesterone level, 3-5ml of blood is extracted every day, the serum is obtained through a centrifuge, and then the DSL-3900 Active Progesterone Coated-Tube Radioimmunoassay Kit (DSL-3900 ACTIVE Progesterone Coated-Tube Radioimmunoassay Kit) is used (diagnostics) System Laboratories Inc....
Embodiment 2
[0073] Enucleation of recipient oocytes
[0074] Without Ca 2+ Add Hepes buffer to the CR2 medium to make hCR2aa medium (see Table 2), and then add 0.1% (v / v) hyaluronidase (Sigma, USA) (Charles Rosenkrans 2; Rosenkrans et al., Biol. Reprod) .49, 459-462, 1993). Then, the cumulus cells of the oocytes obtained in Example 1 were removed by repeatedly using a pipette in the medium prepared above. The oocytes were stained with 5 μg / mL benzimine (Hoechst 33342) for 5 minutes, and then observed with an inverted epi-fluorescence microscope at 200 times magnification to select the oocytes containing the first polar body. Add 10% (v / v) FBS and 5μg / ml cytochalasin B to the hCR2aa medium (see Table 3), and then use a micromanipulator (Narishige, Tokyo, Japan) in the culture medium to enucleate the selected oocytes . That is, the oocytes are placed in a micropipette (internal diameter 150μm), and then the first polar body, adjacent cytoplasm (less than 5%) and the oocyte nucleus are removed...
Embodiment 3
[0082] Donor cell production
[0083] Extract adult fibroblasts from dogs as nucleus donor cells. To this end, the ear skin of a male Afghan hound (2 months old) was first isolated for biopsy. Some small fragments in the ear tissue fragments were washed 3 times with DPBS (Dulbecco's phosphate buffered saline) and then minced with a surgical scalpel. The minced tissue was placed in Dulbecco's modified Eagle's medium containing 0.25% (w / v) insulin and 1 mM EDTA and dissociated at 37°C for 1 hour. Put the enzyme-hydrolyzed cells in Ca-free 2+ And Mg 2+ -Wash once in DPBS, centrifuge at 300xg for 2 minutes, and then plant in a 60-mm plastic petri dish (Becton Dickinson, Lincoln Park, NJ). The seeded cells were then put into the supplemented with 10% (v / v) FBS, 1mM glutamate, 25mM NaHCO 3 And 1% (v / v) minimal essential medium (MEM) non-essential amino acid solution (Invitrogen, CA) in DMEM at 39°C, 5% CO 2 Cultivate for 6-8 days under the condition of 95% air. After removing the non-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com