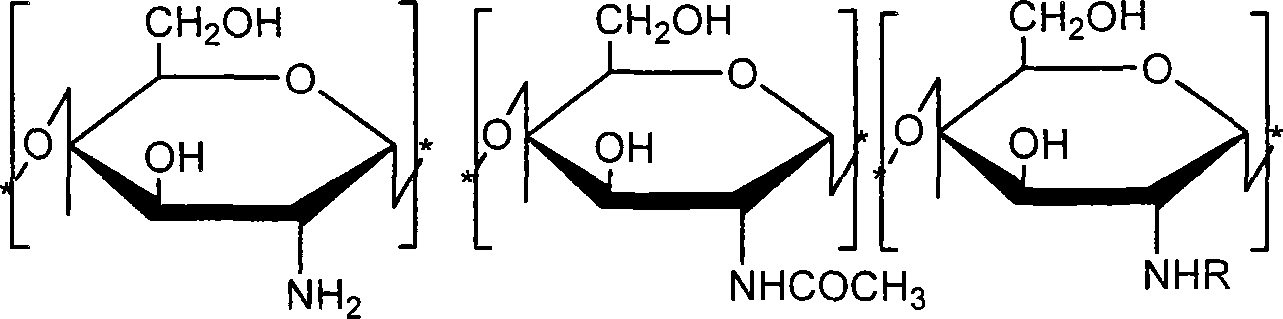
Water-soluble chitosan derivatives as well as preparation method and uses thereof
A technology of water-soluble chitosan and derivatives, applied in medical science, prosthesis, etc., can solve the problems of intense reaction conditions, many reaction steps, and low yield, and achieve the effect of simple operation process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Add 68.75g of acryloyl chloride dropwise to 500ml of toluene solution containing 76.88g of triethylamine and 89.88g of hydroxyethyl methacrylate, and react at 0-5°C. After 4 hours of reaction, the reaction is terminated , washed with 105 ml of water, 100 ml of 1M hydrochloric acid, and 100 ml of 1M NaOH aqueous solution to remove unreacted substances and by-products to obtain 90.01 g of acryloyloxyethyl methacrylate.
[0023] (2) At normal temperature, 6.0 g of chitosan raw material (viscosity-average molecular weight is 150,000, deacetylation degree is 85%) is dissolved in 200 ml of aqueous solution of 2.4 ml glacial acetic acid, then 200 ml of ethanol is added, stirred evenly, and then Add 5.64g of acryloyloxyethyl methacrylate and react at 50°C. After 48 hours of reaction, dialyze to remove unreacted substances and by-products, and freeze-dry to obtain methacryloyloxyethyl carboxyethyl shell Polysaccharide 5.2g, its structural formula is as follows:
[0024] ...
Embodiment 2
[0032] (1) Add 68.75g of acryloyl chloride dropwise to 500ml of toluene solution containing 76.88g of triethylamine and 89.88g of hydroxyethyl methacrylate, and react at 0-5°C. After 4 hours of reaction, the reaction is terminated , washed with 105 ml of water, 100 ml of 1M hydrochloric acid, and 100 ml of 1M NaOH aqueous solution to remove unreacted substances and by-products to obtain 90.01 g of acryloyloxyethyl methacrylate.
[0033] (2) At normal temperature, 6.0 g of chitosan raw material (viscosity-average molecular weight is 150,000, deacetylation degree is 85%) is dissolved in 200 ml of aqueous solution of 2.4 ml glacial acetic acid, then 200 ml of ethanol is added, stirred evenly, and then Add 11.28g of acryloyloxyethyl methacrylate and react at 50°C. After 48 hours of reaction, dialyze to remove unreacted substances and by-products, freeze-dry to obtain methacryloyloxyethyl carboxyethyl shell Polysaccharide 5.1g, its structural formula is as follows:
[0034]
[...
Embodiment 3
[0042](1) Add 68.75g of acryloyl chloride dropwise to 500ml of toluene solution containing 76.88g of triethylamine and 89.88g of hydroxyethyl methacrylate, and react at 0-5°C. After 4 hours of reaction, the reaction is terminated , washed with 105 ml of water, 100 ml of 1M hydrochloric acid, and 100 ml of 1M NaOH aqueous solution to remove unreacted substances and by-products to obtain 90.01 g of acryloyloxyethyl methacrylate.
[0043] (2) At normal temperature, 6.0 g of chitosan raw material (viscosity-average molecular weight is 150,000, deacetylation degree is 85%) is dissolved in 200 ml of aqueous solution of 2.4 ml glacial acetic acid, then 200 ml of ethanol is added, stirred evenly, and then Add 16.92g of acryloyloxyethyl methacrylate and react at 50°C. After 48 hours of reaction, dialyze to remove unreacted substances and by-products, freeze-dry to obtain methacryloyloxyethyl carboxyethyl shell Polysaccharide 5.1g, its structural formula is as follows:
[0044]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com