Dual functional polymer nanometer glue bunch and preparing method and application in preparing medicine for treating second narrow blood vessel
A nano-micelle and polymer technology, applied in the preparation of drugs for the treatment of vascular restenosis, bifunctional polymer nano-micelle and its preparation field, can solve the problem of short retention time, lack of tissue and organ targeting, and drug /Problems such as low bioavailability of gene nanoparticles to achieve high-efficiency treatment, best therapeutic effect, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of bifunctional polymer nanomicelle is made by following method:
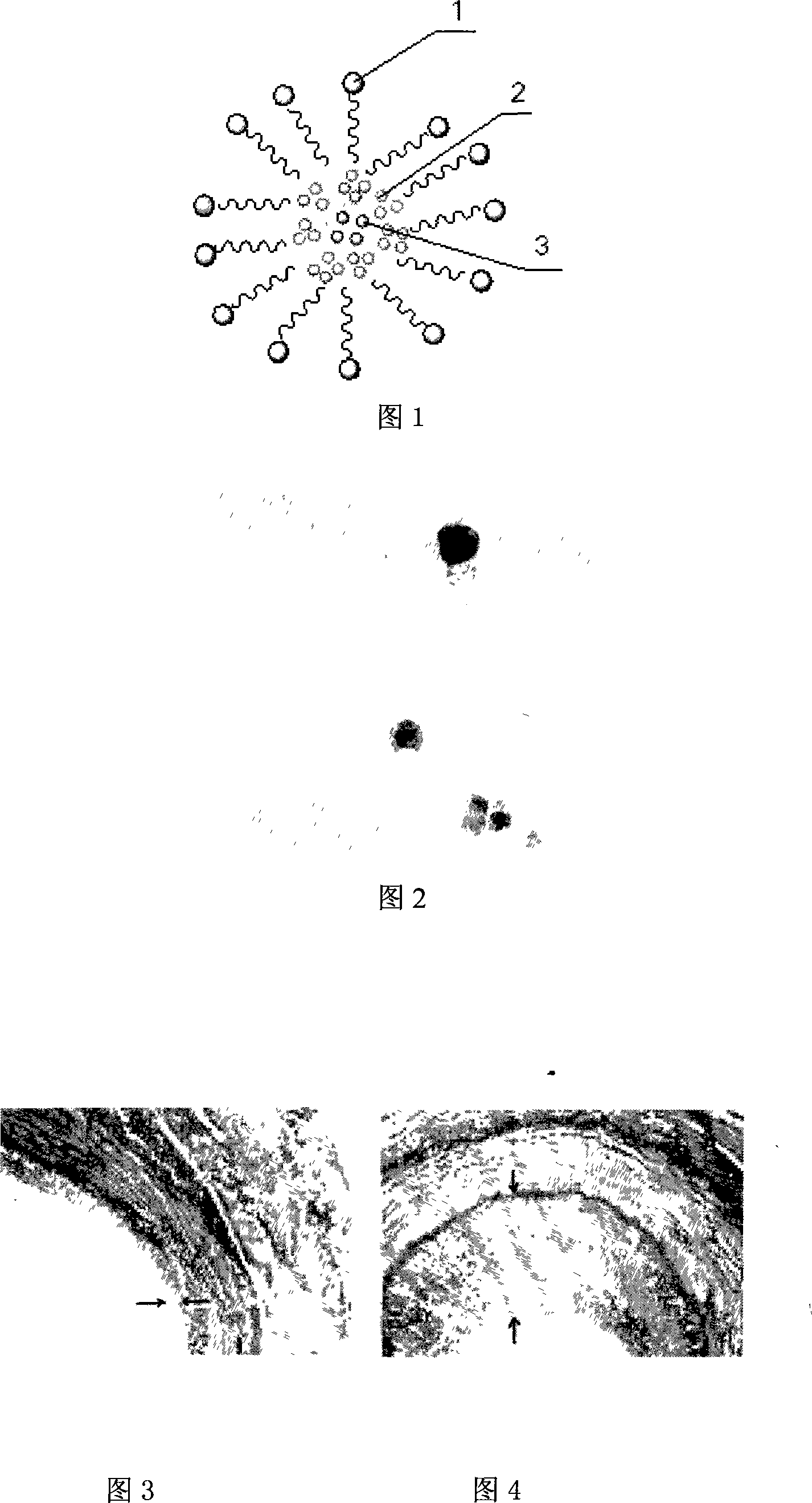
[0037] (1) Mix PLGA / PEG and GPIIb / IIIa with a molar ratio of 1:1, and react for 18 hours at 35° C. under the catalysis of DCC, so that GPIIb / IIIa is connected to PLGA / PEG through an amide bond, and the DCC and The molar ratio of PLGA / PEG is 0.01:1; the amount of binding target molecules is monitored by titration method; NMR, mass spectrometry, infrared and elemental analysis, GPC and other means are used to verify the reaction degree and yield of each step of the reaction, and the purity of the product , the degree of substitution of the terminal group, the content of the terminal amino group, etc. Using surface infrared spectroscopy and multifunctional electron spectroscopy (XPS) to measure the surface functional group content of nanomicelles, thereby determining the surface GPIIb / IIIa content;
[0038] (2) the aqueous solution of the acidic fibroblast growth factor that concentration is 1mg / ml ...
Embodiment 2
[0042] A kind of bifunctional polymer nanomicelle is made by following method:
[0043](1) Mix PLA / PEG with a molar ratio of 1:1.5 and GPIIb / IIIa, and react for 48 hours at 30° C. under the catalysis of DCC, so that GPIIb / IIIa is connected to PLA / PEG through an amide bond, and the DCC and The molar ratio of PLA / PEG is 0.001:1;
[0044] (2) the aqueous solution of basic fibroblast growth factor that concentration is 0.001mg / ml is injected into the acetone solution of the product prepared in step (1) that concentration is 1000mg / ml by volume ratio, mixes 2 hours , into a homogeneous suspension;
[0045] (3) adding the acetone solution of the product prepared in step (1) with a concentration of 1000 mg / ml into the acetone solution of paclitaxel with a concentration of 10 mg / ml in a ratio of 1:50 by volume;
[0046] (4) Add the solution prepared in step (3) to the homogeneous suspension prepared in step (2) according to the specific volume ratio of 5:1, stir for 6 hours, and dia...
Embodiment 3
[0048] A kind of bifunctional polymer nanomicelle is made by following method:
[0049] (1) Mix PGA / PEG and GPIIb / IIIa with a molar ratio of 1:5, catalyzed by DCC, react for 4 hours at 37° C., so that GPIIb / IIIa is connected to PGA / PEG through an amide bond, and the DCC and The molar ratio of PGA / PEG is 0.05: 1;
[0050] (2) the aqueous solution of the epidermal growth factor that concentration is 100mg / ml is injected into the dichloromethane solution that concentration is the product that step (1) prepares of 0.01mg / ml by volume ratio, mix 1 hour, into a homogeneous suspension;
[0051] (3) the dichloromethane solution of the product prepared by the step (1) that the concentration is 100mg / ml is added to the dichloromethane solution of the matrix metalloproteinase inhibitor with a concentration of 100mg / ml in a ratio of 1: 20 by volume middle;
[0052] (4) According to the specific volume ratio of 50~0.1:1, the solution prepared in step (3) was added to the homogeneous sus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com