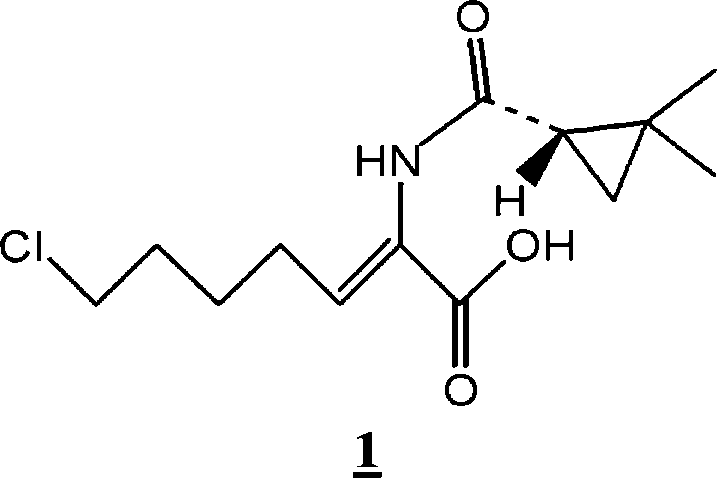
Preparation method for (Z)-7-chloro-((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid
A technology of dimethylcyclopropaneamide and heptenoic acid, which is applied in the field of key intermediates of cilastatin, and can solve the problem that the mixture is difficult to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ( 1 ) preparation
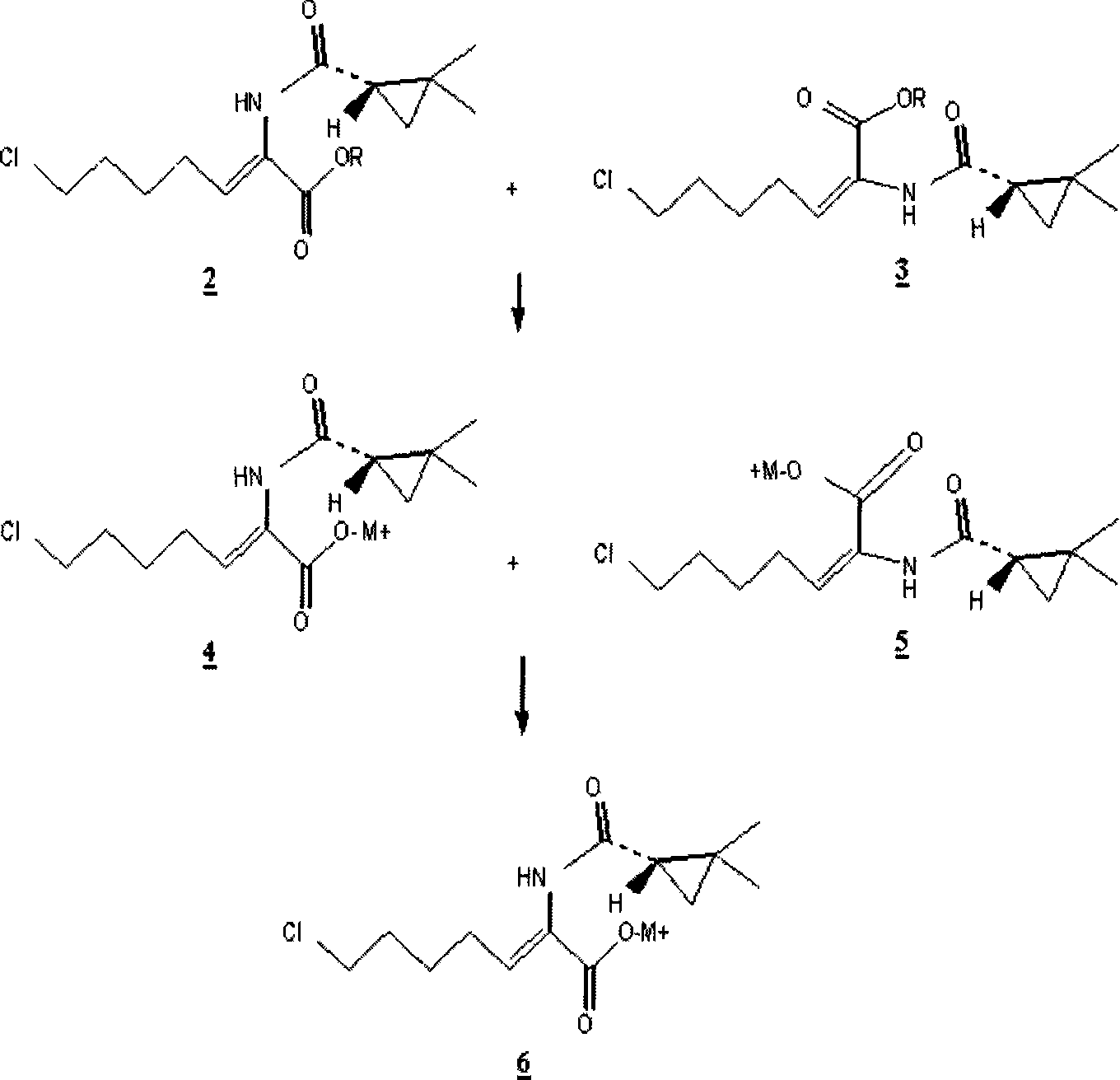
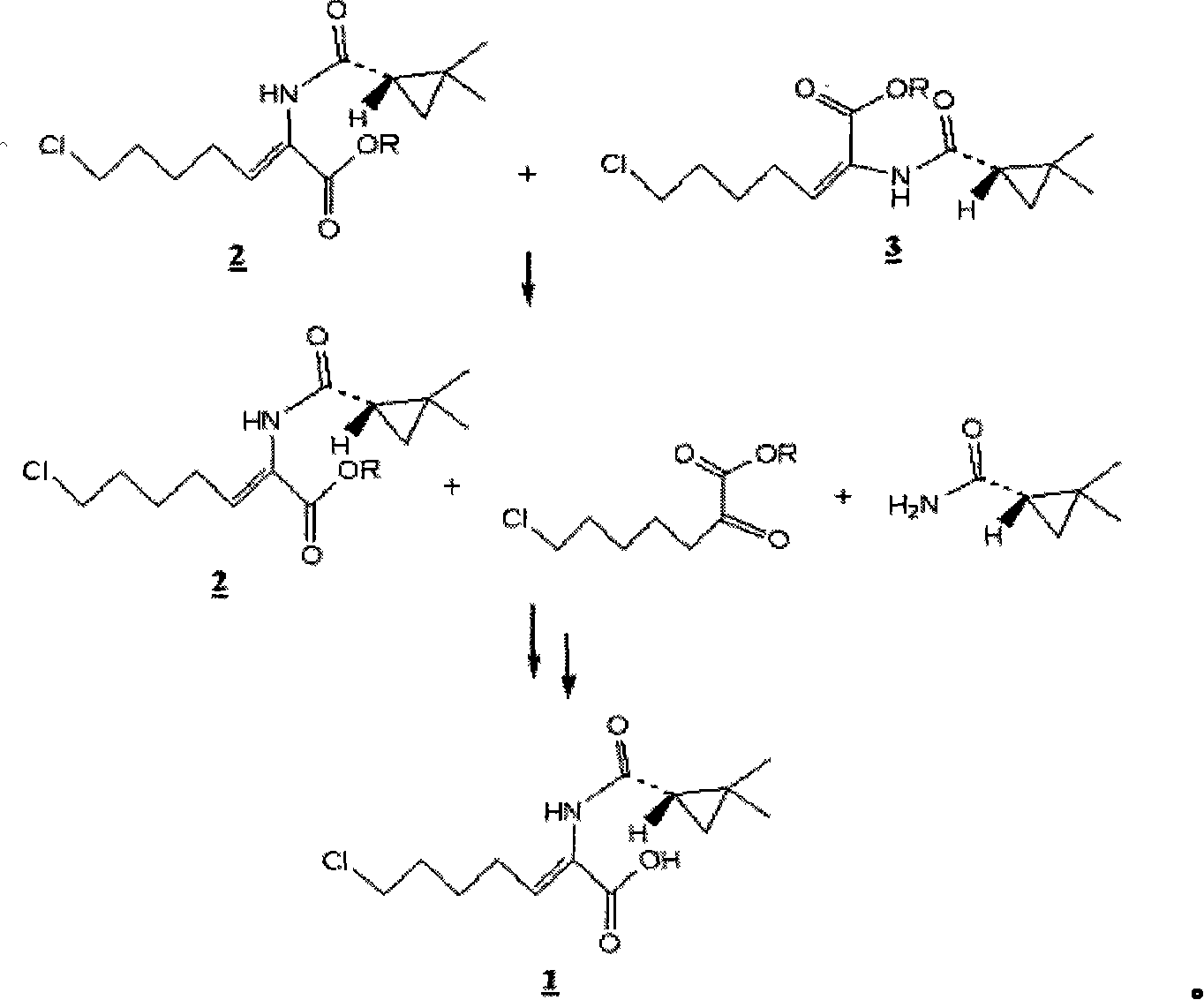
[0031] Get 200g (0.66mol) (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester ( 2 ), dissolved in 600ml of dichloromethane. 126 g of 4-toluenesulfonic acid was added to the mixture and stirred at 20°C. When analyzed according to gas chromatography, ethyl (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoate ( 2 ) and (E)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester ( 3 ) at a (molar) ratio of about 200:1, the pH of the mixture was adjusted to 8-9 by adding 0.5N caustic soda solution and the organic phase was separated. The separated organic phase was concentrated under reduced pressure and added to 1,000 ml of acetonitrile. Next, add 1,420ml (0.925mol) of 0.65N caustic soda solution and stir at room temperature until (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2- Ethyl heptenoate ( 2 ) fully reacted...
Embodiment 2
[0034] Embodiment 2 (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ( 1 ) preparation
[0035] Get 100g (0.33mol) (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester ( 2 ), dissolved in 300ml toluene. 47 g of methanesulfonic acid was added to the mixture and stirred at 30°C. When analyzed according to gas chromatography, ethyl (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoate ( 2 ) and (E)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester ( 3 ) at a (molar) ratio of about 120:1, the reaction was terminated and the mixture was stirred. The pH of the mixture was adjusted to 8-9 by adding 0.5N caustic soda solution and the organic phase was separated. The separated organic phase was concentrated under reduced pressure and added to 300 ml of ethanol. Next, add 380ml (0.46mol) of 1.2N caustic soda solution and stir at room temperature until (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptyl...
Embodiment 3
[0038] The preparation of embodiment 3 cilastatin aqueous solution
[0039] Get (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid obtained in 34.4g embodiment 1 ( 1 ), be dissolved in the mixed aqueous solution of L-cysteine hydrochloride (20g) and caustic soda solution (116ml), stir at room temperature. The reaction was continued until (Z)-7-chloro-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester according to high-speed liquid chromatography analysis ( 2 ) is completely consumed. As a result, a highly pure aqueous solution of cilastatin containing only a trace amount of the E isomer was obtained.
[0040] The method of removing isomeric impurities is very important in the preparation of key supplements of cilastatin-antibiotics such as imipenem. From this point of view, the process of the present invention is useful industrially, because the simple selective acid hydrolysis removes the A large amount of E isomer impurities in the preparat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com