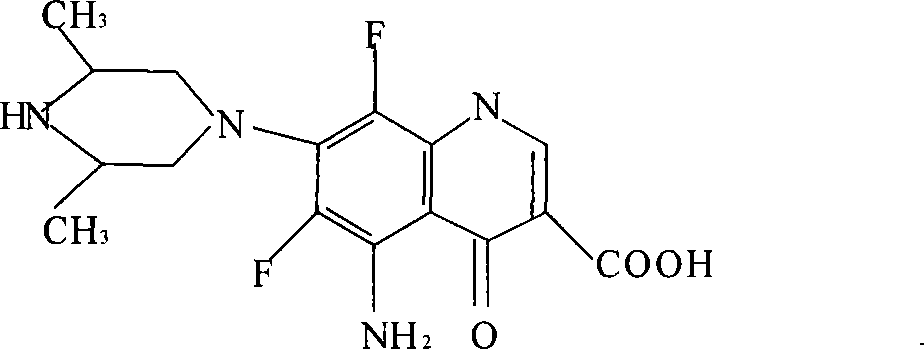
Water-soluble salt of aspartic acid carbostyril series antibacterial drugs and injection dosage forms thereof
A technology of aspartate quinolone and aspartate quinolone, which is applied in the field of injection dosage forms, can solve the problems of nosocomial infection, insufficient understanding of antibiotic side effects, abuse of prescription drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 0.01mol of sparfloxacin, 0.01mol of L-aspartic acid and 40ml of water into a 100ml beaker, stir at room temperature for 10min, the raw materials dissolve rapidly, the resulting solution is a transparent liquid, measure the pH value of the solution with a pH meter is 6.2. Concentrate the obtained salt solution under reduced pressure, then transfer it to a petri dish and place it in a desiccator, let it volatilize the water, after the water is removed, it becomes a transparent glassy solid, and then continue to place it to let the water evaporate completely, gradually turning into a yellow solid , The obtained product weighs 5.20g. The product is the water-soluble salt of the quinolone drugs of the present invention. Add 30ml of water to the solid, shake it, and within about 3 minutes, the solid is completely dissolved.
Embodiment 2
[0029] Take 1.34 g of sparfloxacin L-aspartate prepared in Example 1, add 9.0 g of sodium chloride and add water to 1000 ml to dissolve, add 0.1% charcoal for needles, stir at room temperature for 15 min, filter, pass through 0.2 μm Microporous filter membrane, filled in a colorless infusion bottle, 100ml / bottle, made into an infusion according to the routine, to get final product.
Embodiment 3
[0031] Take 1.34 g of sparfloxacin L-aspartate prepared in Example 1, add water to 20 ml, dissolve, add 0.1% charcoal for needles, stir at room temperature for 15 min, filter, pass through a 0.2 μm microporous filter membrane, pour Packed in colorless glass ampoules, 2ml / branch, made into injection solution according to routine, that is to say.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com