Solid lipid nanometer particle in-situ gel rubber preparations of biological adherent cyclosporine A and preparation method thereof
A solid lipid nanometer and bioadhesion technology, applied in the field of medicine, can solve the problems of short action time, low viscosity, prolonged residence time, etc., and achieve the effects of increasing stability, reducing dosage and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] According to the composition of Example 1 given in Table 1 below, a mixture of 400 mg of glyceryl monostearate and 100 mg of glyceryl behenate was melted in a water bath at 80°C, and 40 mg of cyclosporin A and 600 mg of poloxamer 188 were added successively. After stirring and mixing evenly, add 7.5ml of deionized water at the same temperature dropwise, and stir for 10 minutes until colostrum is formed. Colostrum was sonicated at 400w for 6 minutes, passed through a 0.45um microporous membrane while it was still hot, and the pre-prepared octadecylamino acid solution was added dropwise, continued sonication for half a minute, and cooled at room temperature.
[0045] Preparation of octadecylamine solution: dissolve 340 mg of octadecylamine in 0.5 ml of hot absolute ethanol, add 2 ml of 5% dilute hydrochloric acid, and shake well.
[0046] Take 2.5ml of the above nanoparticle suspension, dilute it to 10ml with deionized water, slowly add 2.4g of poloxamer 407 under high-sp...
Embodiment 2-4
[0048] Using the same preparation method as in Example 1, the nanoparticle in-situ gel preparations in Examples 2-4 were prepared from the composition and process parameters given in Table 1.
[0049] Table 1 Example 1-4 Nanoparticle in situ gel preparation composition and preparation process
[0050]
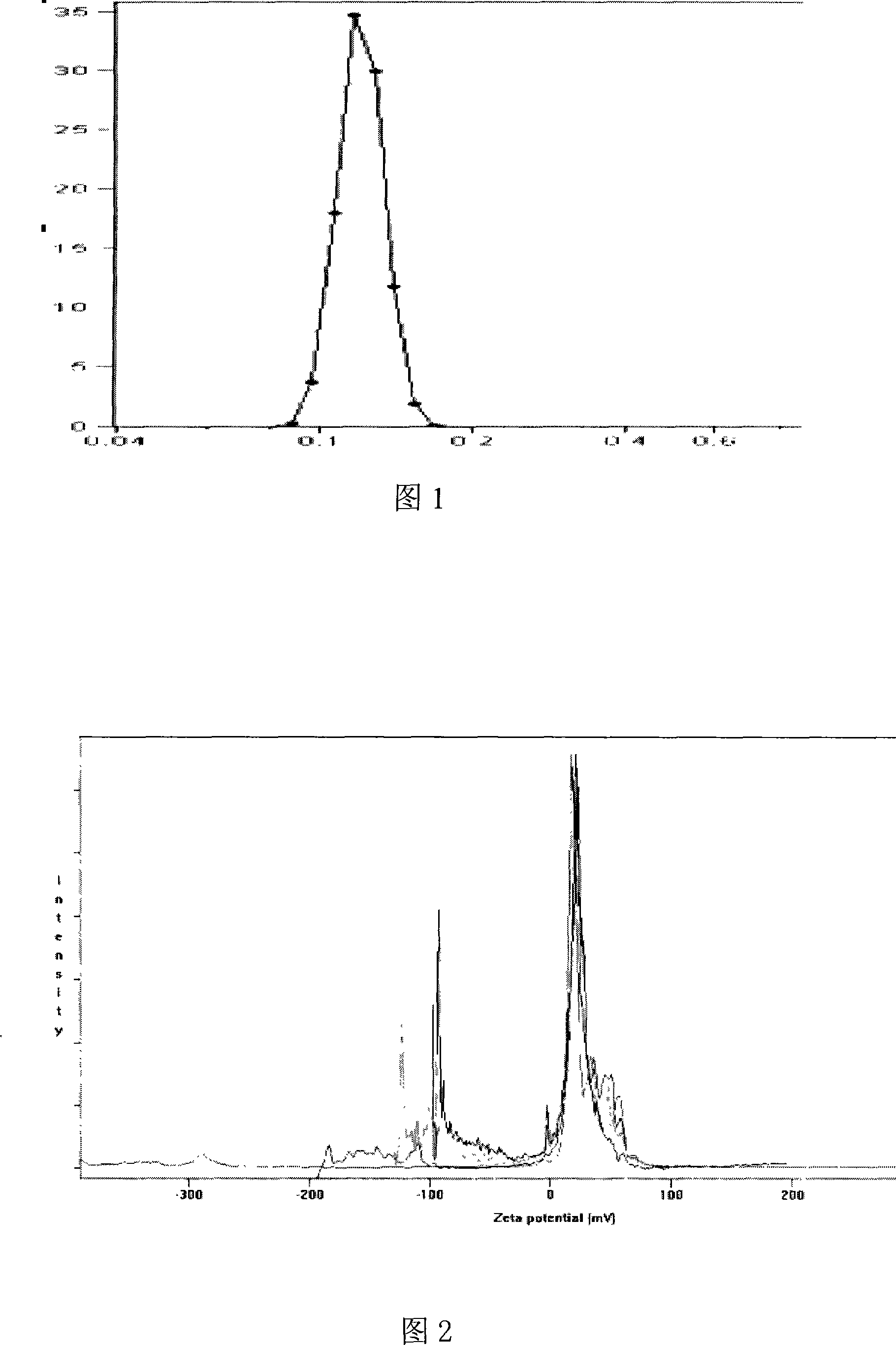
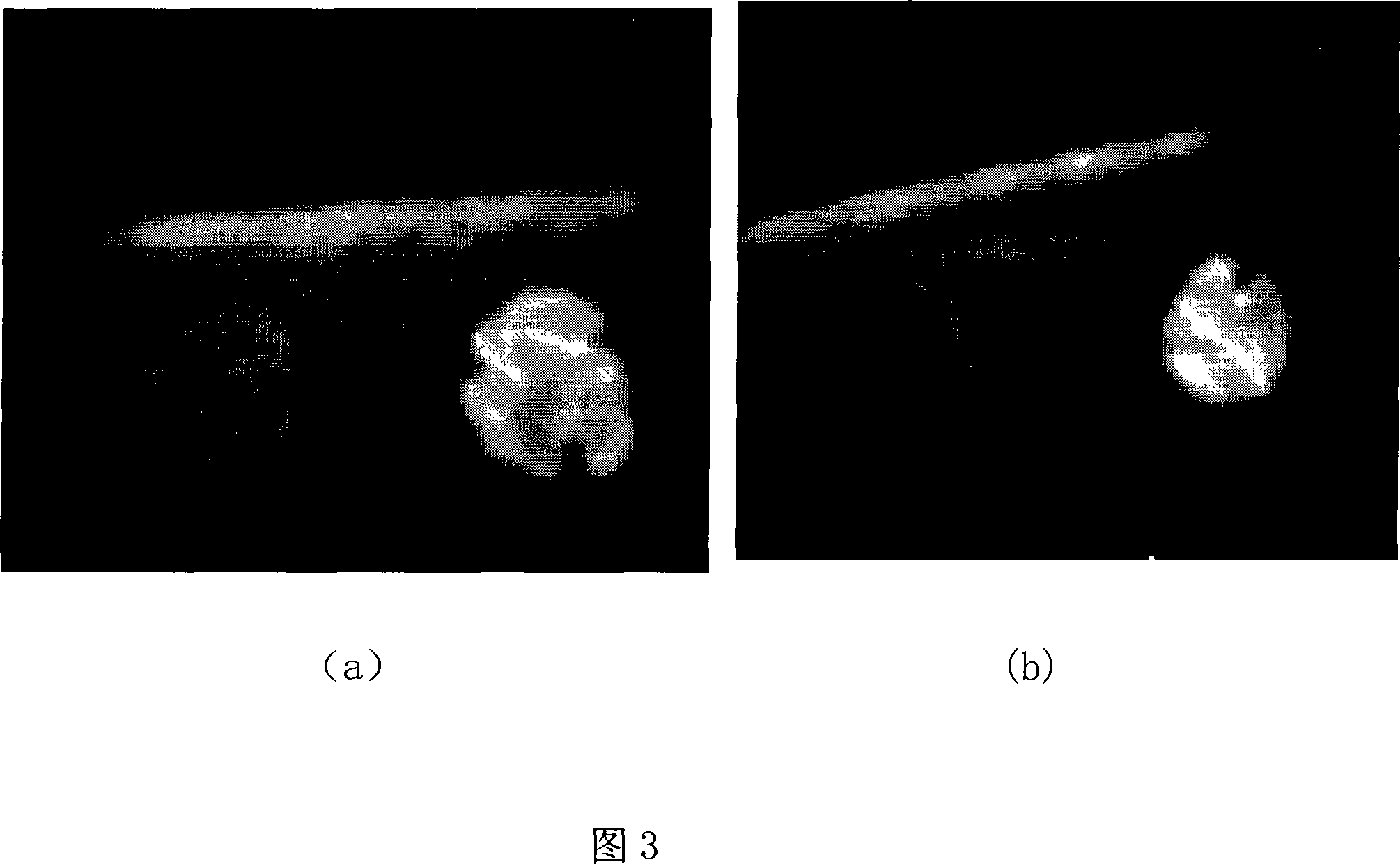
[0051] (1) Determination of particle size distribution and zeta potential of nanoparticles
[0052] The nanoparticle suspension was appropriately diluted with distilled water, the particle size distribution of the sample was measured with a Coulter LS 230, and the potential was measured with a Nicomp-380 / ZLS electrokinetic potential tester.
[0053] (2) Determination of the encapsulation efficiency of cyclosporin A solid lipid nanoparticles
[0054] Cyclosporin solid lipid nanoparticles and free drug were separated by Sephadex G25 dextran microcolumn centrifugation, the contents of cyclosporine in nanoparticles and suspension were determined respectively, and the encapsulat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com