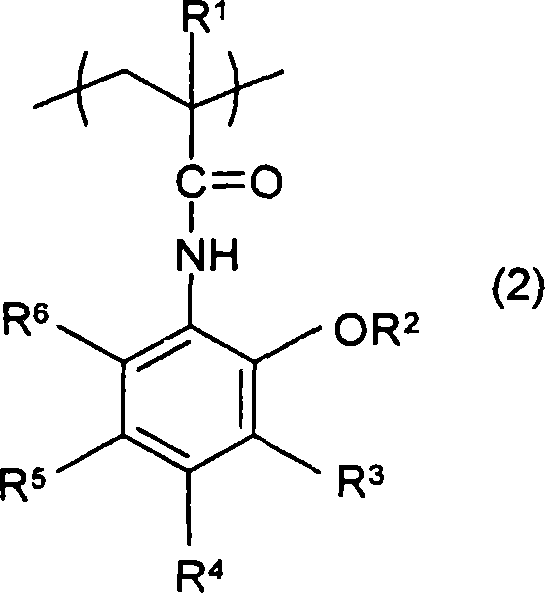
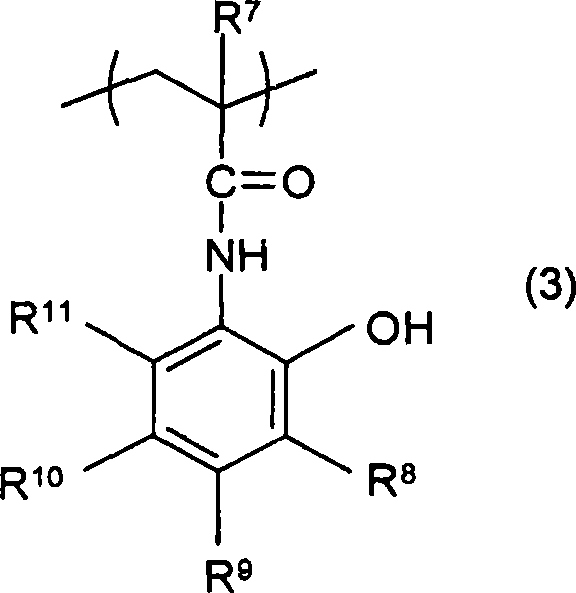
(methyl)acrylamide derivative, polymer, chemically amplified photosensitive resin composition, and method for forming pattern
一种丙烯酰胺、聚合物的技术,应用在丙烯酰胺衍生物,聚合物领域,能够解决正型光敏树脂组合物不是令人满意等问题,达到高分辨率、优异膜性质的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
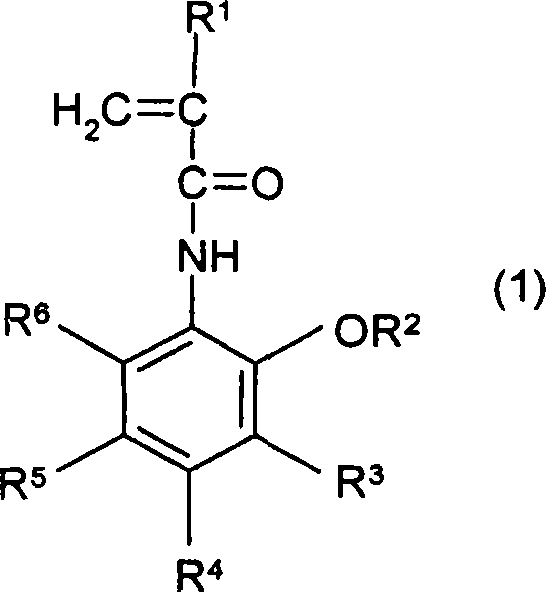
[0227] N-(2-ethoxymethoxyphenyl)acrylamide (acrylamide derivative: A-1 in Table 1; in general formula (1), R 1 is a hydrogen atom, R 2 is ethoxymethyl, R 3 to R 6 is a hydrogen atom) synthetic method
[0228]
[0229] 20 g of N-(2-hydroxyphenyl)acrylamide and 23.76 g of N,N-diisopropylethylamine were dissolved in 150 mL of NMP, and 12.75 g of chloromethyl ethyl ether were added to the solution. The solution was stirred at room temperature. After 3 days, the solution was poured into 1000 mL of water, and the organic layer was extracted with 400 mL of ether. The organic layer was washed successively with 0.2N hydrochloric acid, brine, 3% aqueous sodium hydroxide solution and brine. The organic layer was dried over magnesium sulfate, and then diethyl ether was evaporated under reduced pressure. The residue was recrystallized from hexane / ethyl acetate (100 / 4) to obtain 13.23 g of the desired product (white solid, yield: 49%).
[0230] 1 H-NMR (THF-d 8 , δ): 1.19 (3H, t...
Embodiment 2
[0232] N-(2-(1-ethoxyethoxy) phenyl) acrylamide (acrylamide derivative: A-2 in Table 1; in general formula (1), R 1 is a hydrogen atom, R 2 is ethoxyethyl, R 3 to R 6 is a hydrogen atom) synthetic method
[0233]
[0234] 10 g of N-(2-hydroxyphenyl)acrylamide and 11.05 g of ethyl vinyl ether were dissolved in 50 mL of NMP, and 0.308 g of pyridinium p-toluenesulfonate was added to the solution. The solution was stirred at room temperature. After 6 days, the reaction mixture was poured into 300 mL of water, and the organic layer was extracted with 300 mL of diethyl ether. The organic layer was washed successively with 3% aqueous sodium hydroxide solution and brine. The organic layer was dried over magnesium sulfate, and then the solvent was evaporated under reduced pressure to obtain 9.6 g of the desired product (yield: 67%).
[0235] 1 H-NMR (THF-d 8 , δ): 1.20 (3H, t), 1.53 (3H, d), 3.5-3.8 (2H, m), 5.38 (1H, q), 5.75 (1H, d), 6.29 (1H, dd), 6.41 ( 1H, d), 7.0-7.08...
Embodiment 3
[0237] N-(2-(1-ethoxyethoxy) phenyl) methacrylamide (methacrylamide derivative: A-6 in Table 1; in general formula (1), R 1 is methyl, R 2 is ethoxyethyl, R 3 to R 6 is a hydrogen atom) synthetic method
[0238]
[0239] 10 g of N-(2-hydroxyphenyl)methacrylamide and 10.17 g of ethyl vinyl ether were dissolved in 50 mL of NMP, and 0.284 g of pyridinium p-toluenesulfonate was added to the solution. The solution was stirred at room temperature. After 6 days, the reaction mixture was poured into 300 mL of water, and the organic layer was extracted with 300 mL of diethyl ether. The organic layer was washed successively with 3% aqueous sodium hydroxide solution and brine. The organic layer was dried over magnesium sulfate, and then the solvent was evaporated under reduced pressure to obtain 10.2 g of the desired product (yield: 72%).
[0240] 1 H-NMR (THF-d 8 , δ): 1.18(3H, t), 1.54(3H, d), 2.08(3H, s), 3.49-3.72(2H, m), 5.36(1H, q), 5.47(1H, s), 5.87( 1H, s), 7.0-7.07 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| Weight-average Molecular Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com