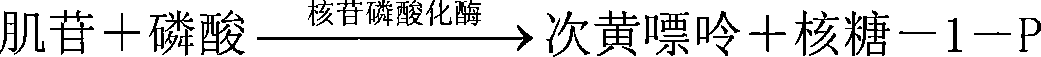Method for determining quality of inosine tablets
A measurement method, the technology of inosine tablets, applied in the direction of measuring devices, electrochemical variables of materials, instruments, etc., can solve the problems of difficult to eliminate interference, high test cost, expensive equipment, etc., achieve short reaction time, reduce cost, and simple method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
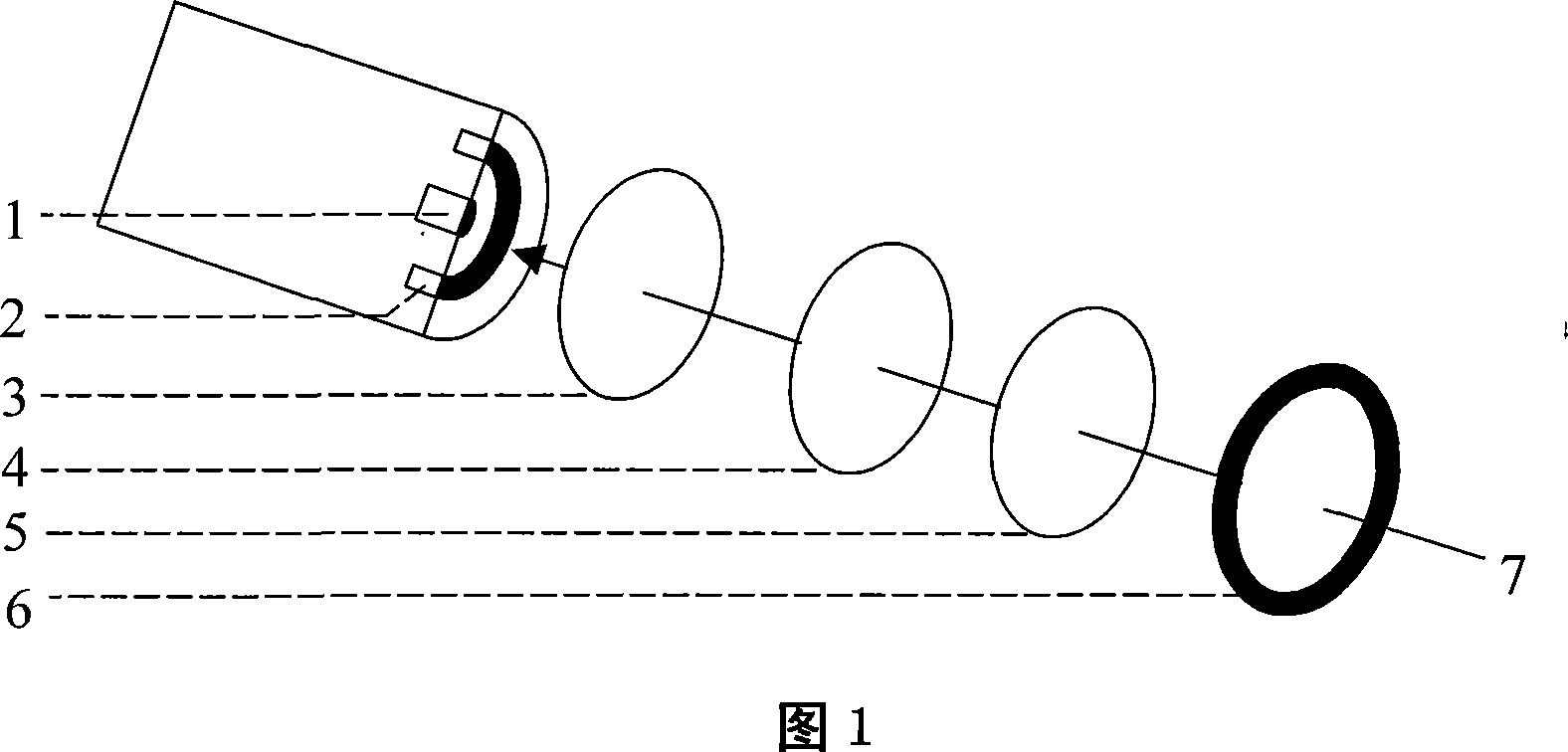
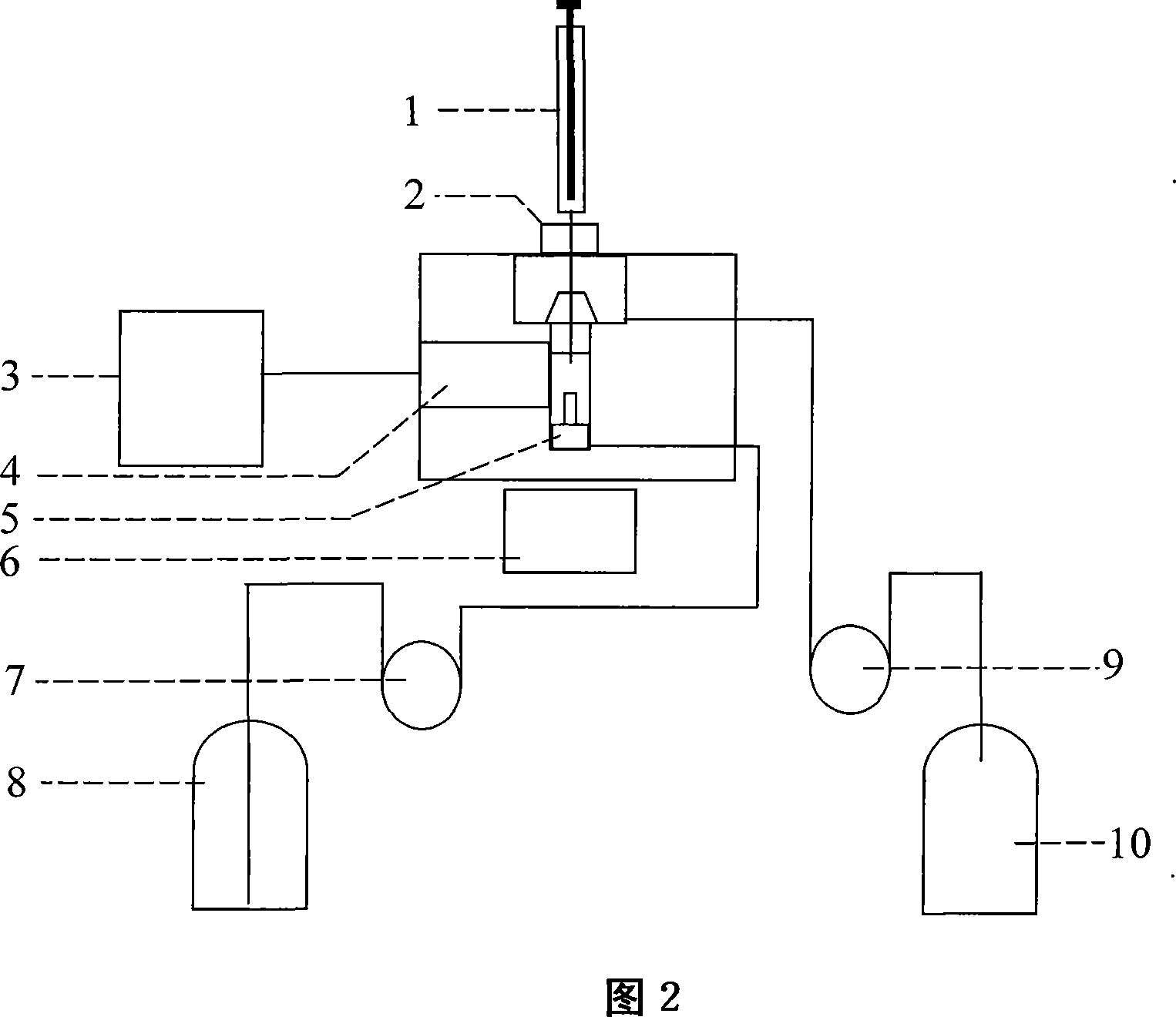
[0028] 1 Instruments and reagents: nucleoside phosphorylase (EC 2.4.2.1), xanthine dehydrogenase (EC 1.2.3.2), inosine reference substance Sigma Company of the United States; bovine serum albumin, glutaraldehyde Shanghai chemical reagent purchase Imported sub-packaging at the supply station; nuclear microporous membrane (0.2 μm) from Nucleopore Company of the United States; platinum and silver purity of 99.999%, Jinan Branch of the People's Bank of China; other reagents are analytically pure. SBA-40 inosine biosensing analyzer.
[0029] 2 Determination principle: The determination of inosine by immobilized double-enzyme composite enzyme membrane combined with hydrogen peroxide electrode is based on the following reaction:
[0030]
[0031]
[0032]
[0033]
[0034] 3 Preparation of inosine reference substance solution: Accurately weigh 268 mg of inosine reference substance, put it in a 1000ml volumetric flask, add distilled water to dissolve, and const...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com