Substituted cinnamic acid nitrogen-containing derivative having tumor cytotoxic activity
A technology for cinnamic acid and derivatives, which is applied in the field of preparation of substituted cinnamic acid nitrogen-containing derivatives and intermediates thereof, can solve the problems of low selectivity, unsatisfactory, limited general applicability of medicines, etc. Low, good cytotoxic activity, simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
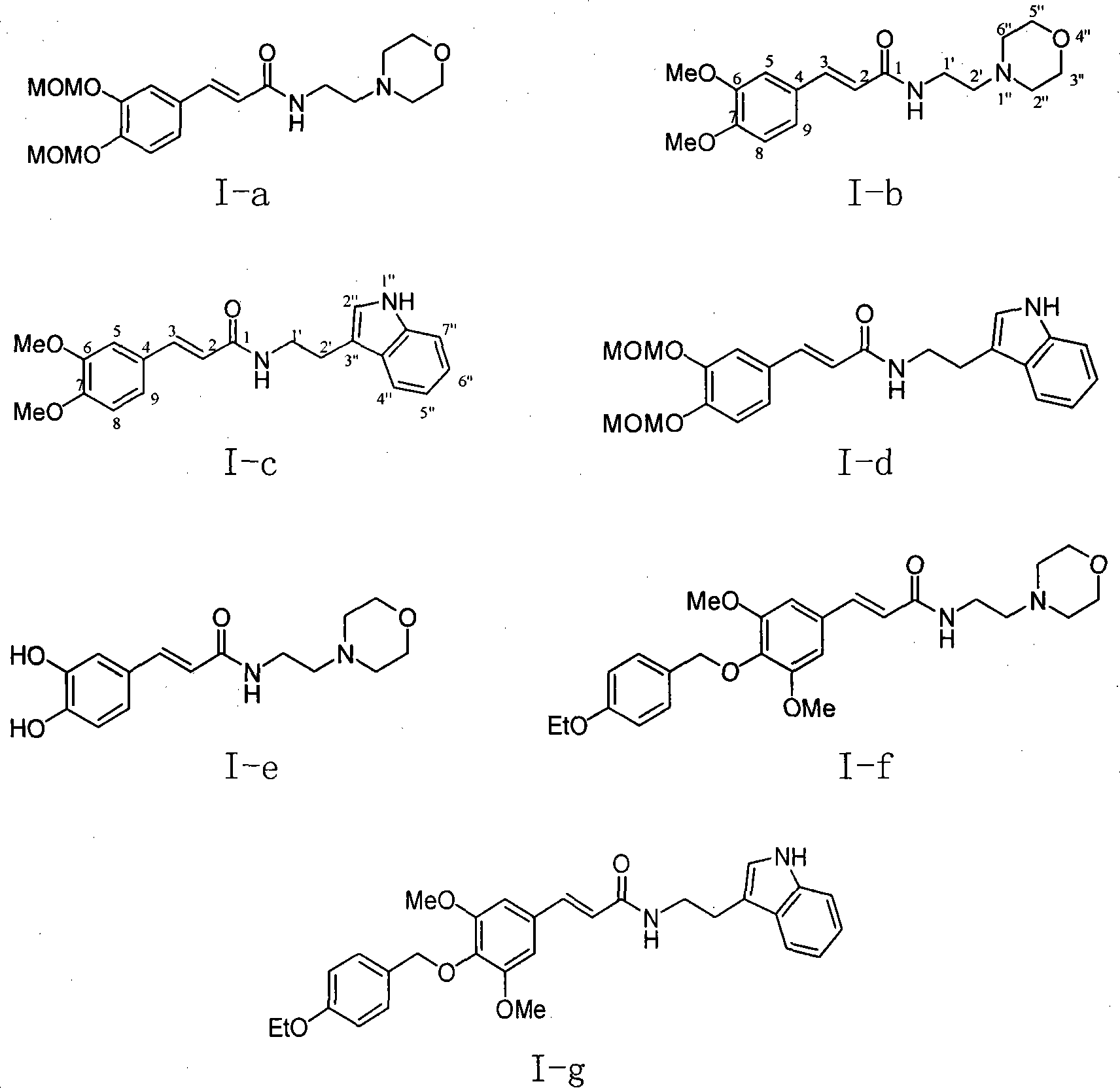
[0037] Example 1 : the preparation of compound I-a i.e. N-(2'-morpholine ethyl)-6,7-dimethoxymethoxycinnamic amide (2E)
[0038]
[0039]This example relates to a class of substituted cinnamon amide derivatives with cytotoxic activity as shown in formula (I) and its key intermediate as N, N'-dicyclohexylguanidine cinnamate shown in formula (II) A general method for the synthesis of derivatives. It specifically relates to the synthesis of compound N-(2'-morpholine ethyl)-6,7-dimethoxymethoxycinnamic amide. The compound 3,4-dimethoxymethoxycinnamic acid (536 mg, 2.0 mmol, obtained from 3,-dihydroxybenzaldehyde through condensation with malonic acid after protection with methoxymethoxy) was dissolved in chloroform , then added dicyclohexylcarbodiimide (DCC, 448 mg, 2.0 mmol), kept stirring at 45 ° C for 1 hour, then added substituted morpholine ethylamine (286 mg, 2.2 mmol), and refluxed for 8 hours Lowered to room temperature, filtered, and the filtrate was concentrated to...
Embodiment 2-10
[0043] According to the method of Example 1, the compounds of Examples 2-10 shown in the following Table 1 were prepared:
[0044]
[0045] Table I
[0046]
[0047] List the physicochemical data of each compound in Table 1 below:
[0048] I-b: Yield: 67.1%; White solid; Rf (dichloromethane / methanol 12:1) 0.49; H NMR 1 H NMR (400MHz, deuterated chloroform CDCl 3 ): δ7.58 (1H, bimodal, J=15.6Hz, H-3), 7.11 (1H, double bimodal, J=8.4, 1.6Hz, H-9), 7.05 (1H, bimodal, J= 1.6Hz, H-5), 6.87(1H, doublet, J=8.4Hz, H-8), 6.31(1H, doublet, J=15.6Hz, H-2), 6.15(1H, broad singlet, NH), 3.93-3.92 (6H, unimodal, CH 3 0-6, 7), 3.75 (4H, triplet, J=4.4Hz, H-3″, 5″), 3.51 (2H, quartet, J=5.6Hz, H-1′), 2.56 (2H , triplet, J=6.0 Hz, H-2'), 2.49 (4H, multiplet, H2", 6").
[0049] I-c: Yield: 53.6%; Pale yellow solid; Rf (dichloromethane / methanol 12:1) 0.66; H NMR 1 H NMR (400MHz, deuterated chloroform CDCl 3 ): δ8.18 (1H, singlet, H-1″), 7.65 (1H, doublet, J=8.0Hz, H-7″), 7.55 (1H, ...
Embodiment 2
[0063] This experiment shows that such substituted cinnamon amide derivatives have strong cytotoxicity to Eca-109 cells, and may be developed into new drugs with anti-esophageal cancer effects. Pharmacological Example 2 : Cytotoxic activity of compound I-d on poorly differentiated gastric adenocarcinoma tumor cells (BGC 823) cells
[0064] Gastric adenocarcinoma tumor cells (BGC 823) were cultured in RPMI 1640 medium containing 5% calf serum, 100 U / ml penicillin and 100 U / ml streptomycin. cells in 4×10 per well 3 The concentration was added to a 96-well plate, and cultured for 24 hours at 37°C in an incubator containing 5% carbon dioxide humidified air.
[0065] Cell viability was determined by the modified MTT method. After the cells were incubated for 24 hours, the newly prepared dimethyl sulfoxide solution of compound I-d was added to each well with a concentration gradient, so that the final concentrations of the compounds in the wells were 100 μg / ml, 33.3 μg / ml, and 11...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com