Substituted cinnamic acid nitrogen-containing derivative having tumor cytotoxic activity
A technology of cinnamon acid and its derivatives, which is applied in the fields of antineoplastic drugs, drug combinations, organic chemistry, etc., can solve the problems of low selectivity, unsatisfactory, and limited general applicability of drugs, and achieves low-cost, high-efficiency synthetic methods Simple, good cytotoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
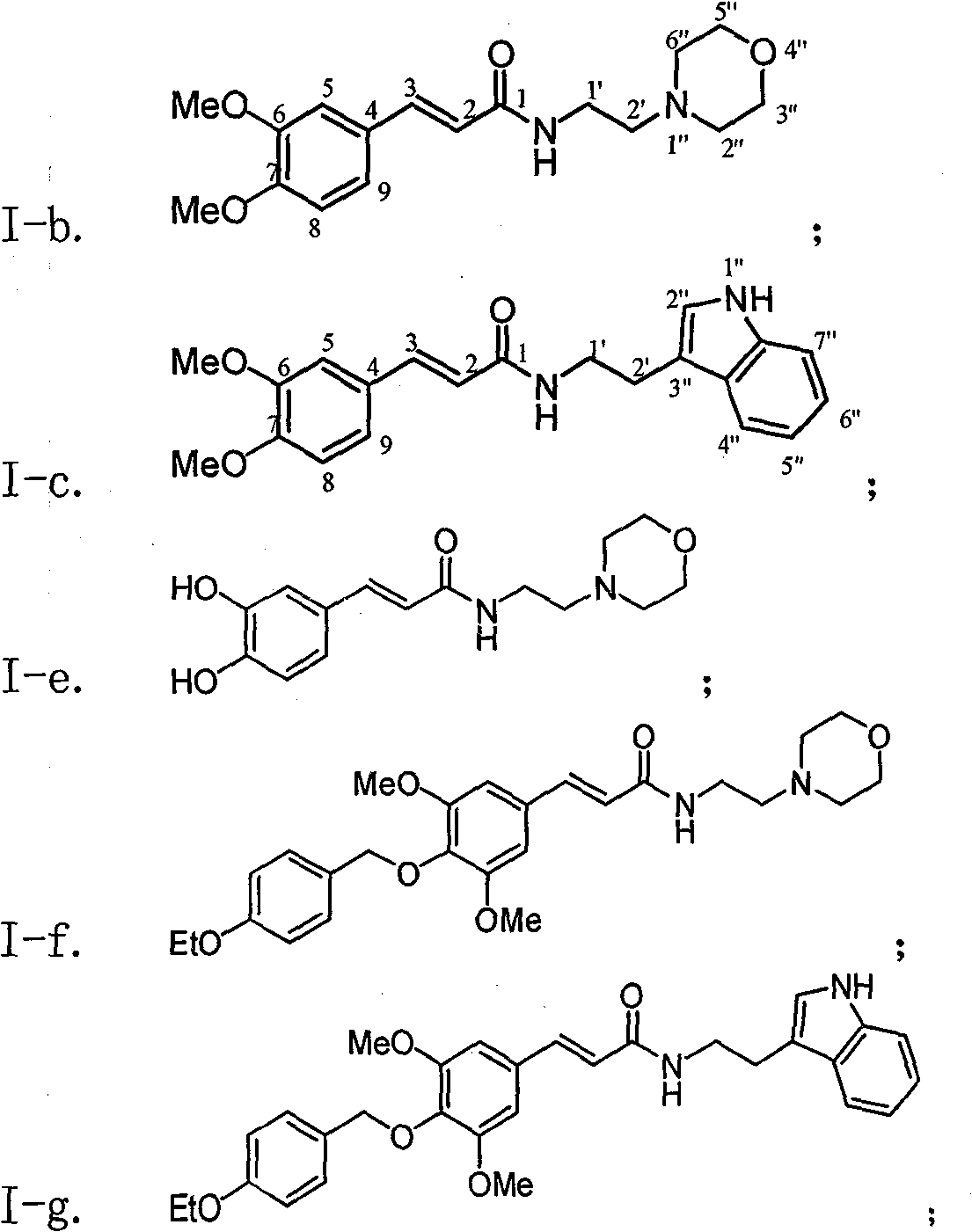
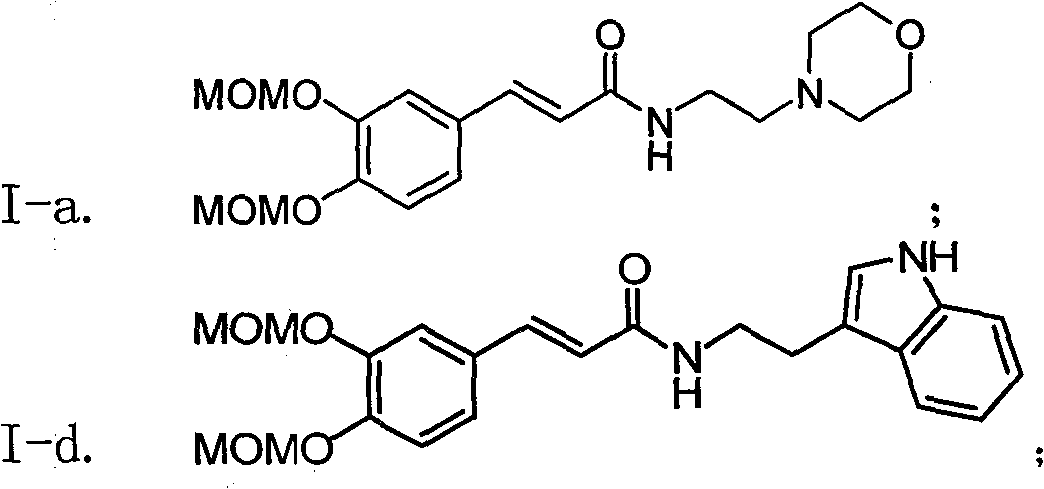
[0037] Example 1 : the preparation of compound I-a i.e. N-(2'-morpholine ethyl)-6,7-dimethoxymethoxycinnamic amide (2E)
[0038]
[0039]This example relates to a class of substituted cinnamon amide derivatives with cytotoxic activity as shown in formula (I) and its key intermediate as N, N'-dicyclohexylguanidine cinnamate shown in formula (II) A general method for the synthesis of derivatives. It specifically relates to the synthesis of compound N-(2'-morpholine ethyl)-6,7-dimethoxymethoxycinnamic amide. The compound 3,4-dimethoxymethoxycinnamic acid (536 mg, 2.0 mmol, obtained from 3,-dihydroxybenzaldehyde through condensation with malonic acid after protection with methoxymethoxy) was dissolved in chloroform , then added dicyclohexylcarbodiimide (DCC, 448 mg, 2.0 mmol), kept stirring at 45 ° C for 1 hour, then added substituted morpholine ethylamine (286 mg, 2.2 mmol), and refluxed for 8 hours Lowered to room temperature, filtered, and the filtrate was concentrated to...
Embodiment 2-10
[0043] According to the method of Example 1, the compounds of Examples 2-10 shown in the following Table 1 were prepared:
[0044]
[0045] Table I
[0046]
[0047] List the physicochemical data of each compound in Table 1 below:
[0048] I-b: Yield: 67.1%; White solid; Rf (dichloromethane / methanol 12:1) 0.49; H NMR 1 H NMR (400MHz, deuterated chloroform CDCl 3 ): δ7.58 (1H, bimodal, J=15.6Hz, H-3), 7.11 (1H, double bimodal, J=8.4, 1.6Hz, H-9), 7.05 (1H, bimodal, J= 1.6Hz, H-5), 6.87(1H, doublet, J=8.4Hz, H-8), 6.31(1H, doublet, J=15.6Hz, H-2), 6.15(1H, broad singlet, NH), 3.93-3.92 (6H, unimodal, CH 3 0-6, 7), 3.75 (4H, triplet, J=4.4Hz, H-3″, 5″), 3.51 (2H, quartet, J=5.6Hz, H-1′), 2.56 (2H , triplet, J=6.0 Hz, H-2'), 2.49 (4H, multiplet, H2", 6").
[0049] I-c: Yield: 53.6%; Pale yellow solid; Rf (dichloromethane / methanol 12:1) 0.66; H NMR 1 H NMR (400MHz, deuterated chloroform CDCl 3 ): δ8.18 (1H, singlet, H-1″), 7.65 (1H, doublet, J=8.0Hz, H-7″), 7.55 (1H, ...
Embodiment 2
[0063] This experiment shows that such substituted cinnamon amide derivatives have strong cytotoxicity to Eca-109 cells, and may be developed into new drugs with anti-esophageal cancer effects. Pharmacological Example 2 : Cytotoxic activity of compound I-d on poorly differentiated gastric adenocarcinoma tumor cells (BGC 823) cells
[0064] Gastric adenocarcinoma tumor cells (BGC 823) were cultured in RPMI 1640 medium containing 5% calf serum, 100 U / ml penicillin and 100 U / ml streptomycin. cells in 4×10 per well 3 The concentration was added to a 96-well plate, and cultured for 24 hours at 37°C in an incubator containing 5% carbon dioxide humidified air.
[0065] Cell viability was determined by the modified MTT method. After the cells were incubated for 24 hours, the newly prepared dimethyl sulfoxide solution of compound I-d was added to each well with a concentration gradient, so that the final concentrations of the compounds in the wells were 100 μg / ml, 33.3 μg / ml, and 11...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com