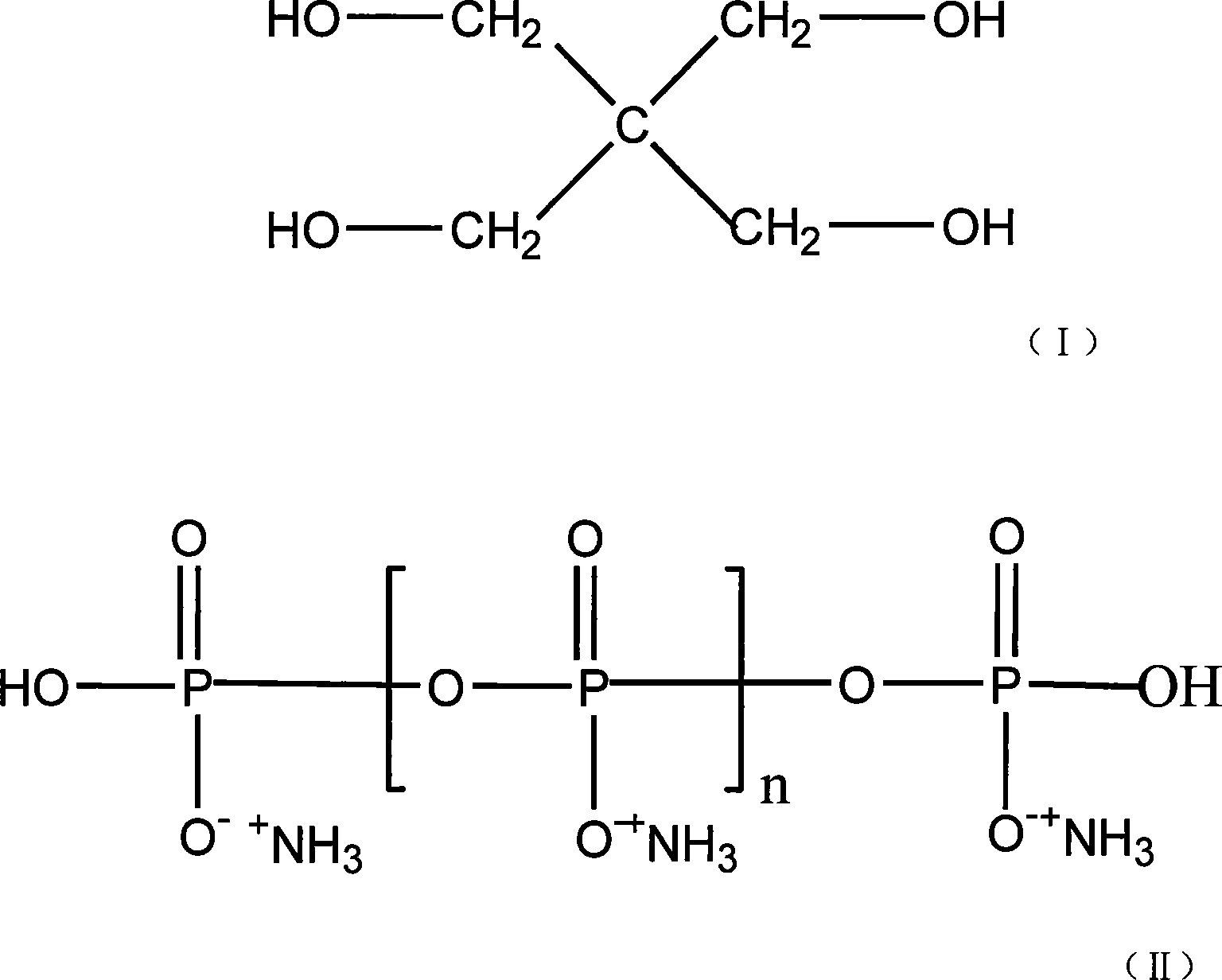
Non-bittern swelling flame-proof polypropylene containing porous nickel phosphate and preparation method thereof
A technology of flame-retardant polypropylene and intumescent flame retardant, which is applied in the field of halogen-free intumescent flame-retardant polypropylene, and can solve the problems that porous nickel phosphate has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] First prepare the synthetic porous nickel phosphate according to the method introduced in "Microporous and Mesoporous Materials" (Microporous and Mesoporous Materials; 85(3):355-364, 2005): Dissolve 106.5g nickel chloride hexahydrate in 314ml deionized In the water, add 55.56g of orthophosphoric acid dropwise under stirring; then add 16.68g of ammonium fluoride; finally add ammonia water to adjust the pH value to 2.5, stir for 0.5 hours, and transfer the mixed material into a polytetrafluoroethylene with a capacity of 500ml In a lined stainless steel synthesis kettle, the filling amount of the mixture is 70% of the volume of the synthesis kettle. Sealed and crystallized at 180°C and autogenous pressure for 144 hours; the obtained yellow-green solid product was washed 3 times with deionized water, first dried in the air, and then dried at 100°C to obtain the product porous nickel phosphate. spare.
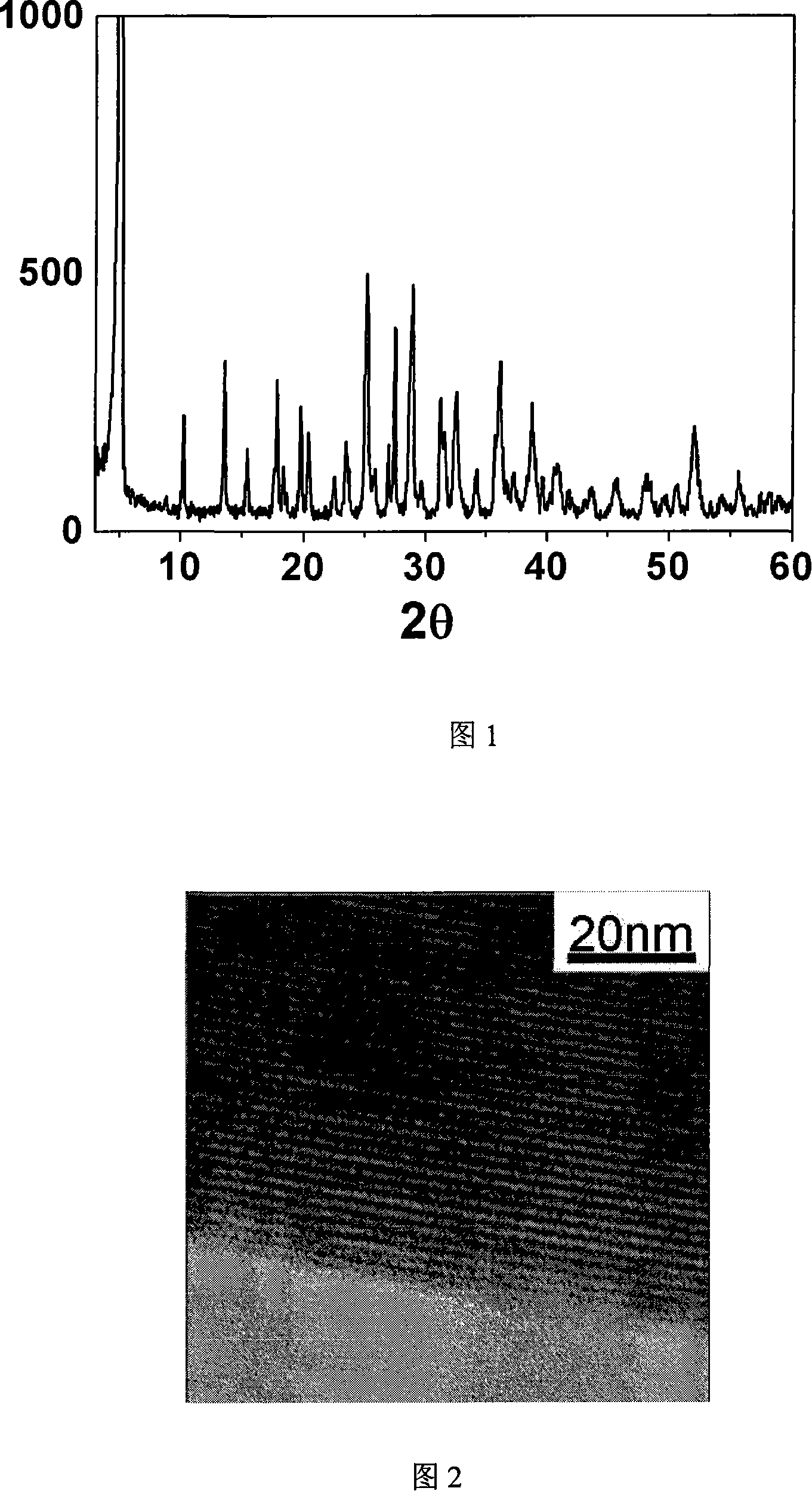
[0020] The porous nickel phosphate obtained above was subjected to X-ra...
Embodiment 2
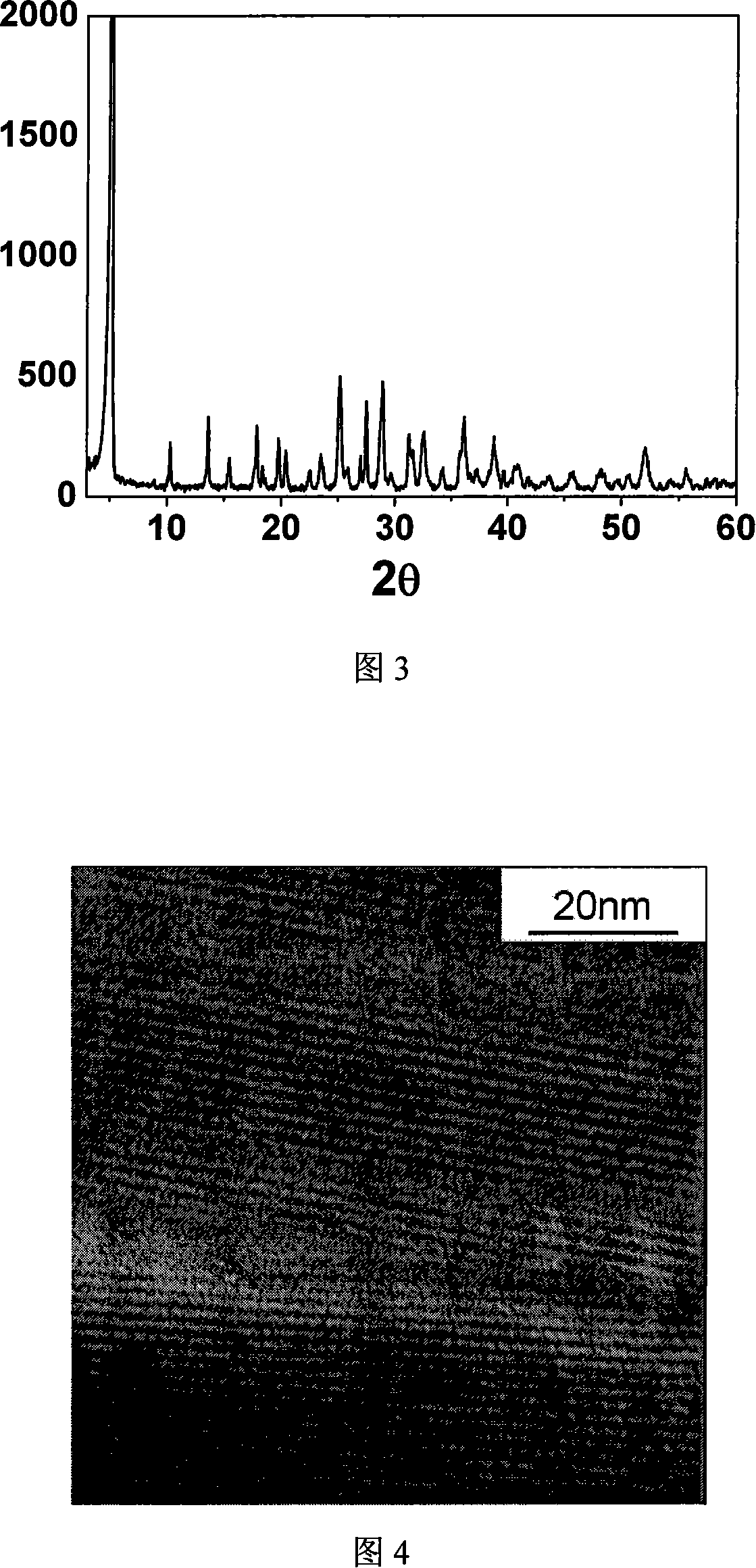
[0027]Dissolve 106.5g of nickel chloride hexahydrate in 314ml of deionized water, add 83.34g of orthophosphoric acid drop by drop under stirring; then add 26.69g of ammonium fluoride; finally add ammonia water to adjust the pH value to 1.8, stir for 0.5 hours after mixing , This mixed material is moved into a capacity of 500ml polytetrafluoroethylene lined stainless steel synthesis kettle, the mixture charge is 70% of the volume of the synthesis kettle. Sealed crystallization at 160°C and autogenous pressure for 72 hours; the obtained yellow-green solid product was washed three times with deionized water, first dried in air, and then dried at 100°C for use. The resulting product was subjected to X-ray diffraction analysis. Fig. 2 is the X-ray diffractogram of the porous nickel phosphate prepared at 160 ℃ of crystallization 72 hours in the present embodiment, same as embodiment Fig. 1, and "Journal of American Chemical Society" (J.Am.Chem.Soc 125: 1309, 2003) and the X-ray dif...
Embodiment 3
[0031] Dissolve 106.5g of nickel chloride hexahydrate in 314ml of deionized water, add 55.56g of orthophosphoric acid dropwise under stirring; then add 16.68g of ammonium fluoride; finally add ammonia water to adjust the pH value to 2.5, and stir for 0.5 hours. This mixed material was moved into a polytetrafluoroethylene-lined stainless steel synthesis kettle with a capacity of 500 ml, and the filling amount of the mixture was 70% of the volume of the synthesis kettle. Sealed crystallization at 190°C and autogenous pressure for 168 hours; the obtained yellow-green solid product was washed three times with deionized water, first dried in air, and then dried at 100°C for use. Figure 4 is a high-resolution electron microscope image of the porous nickel phosphate prepared in this example by crystallization at 190°C for 168 hours, which is consistent with "Microporous and Mesoporous Materials" (Microporous and Mesoporous Materials; 85(3): 355-364, 2005 ) consistent with the high-re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com