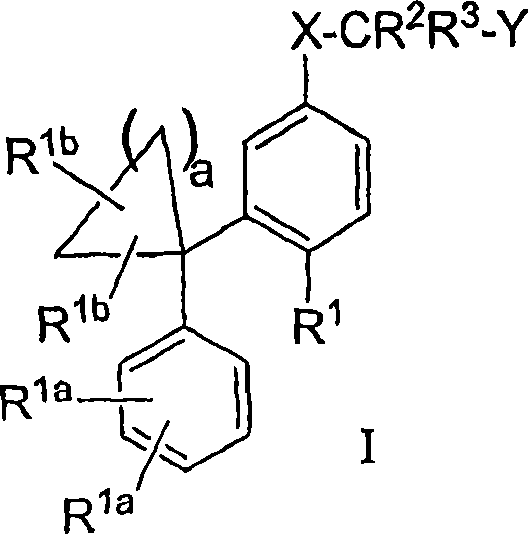
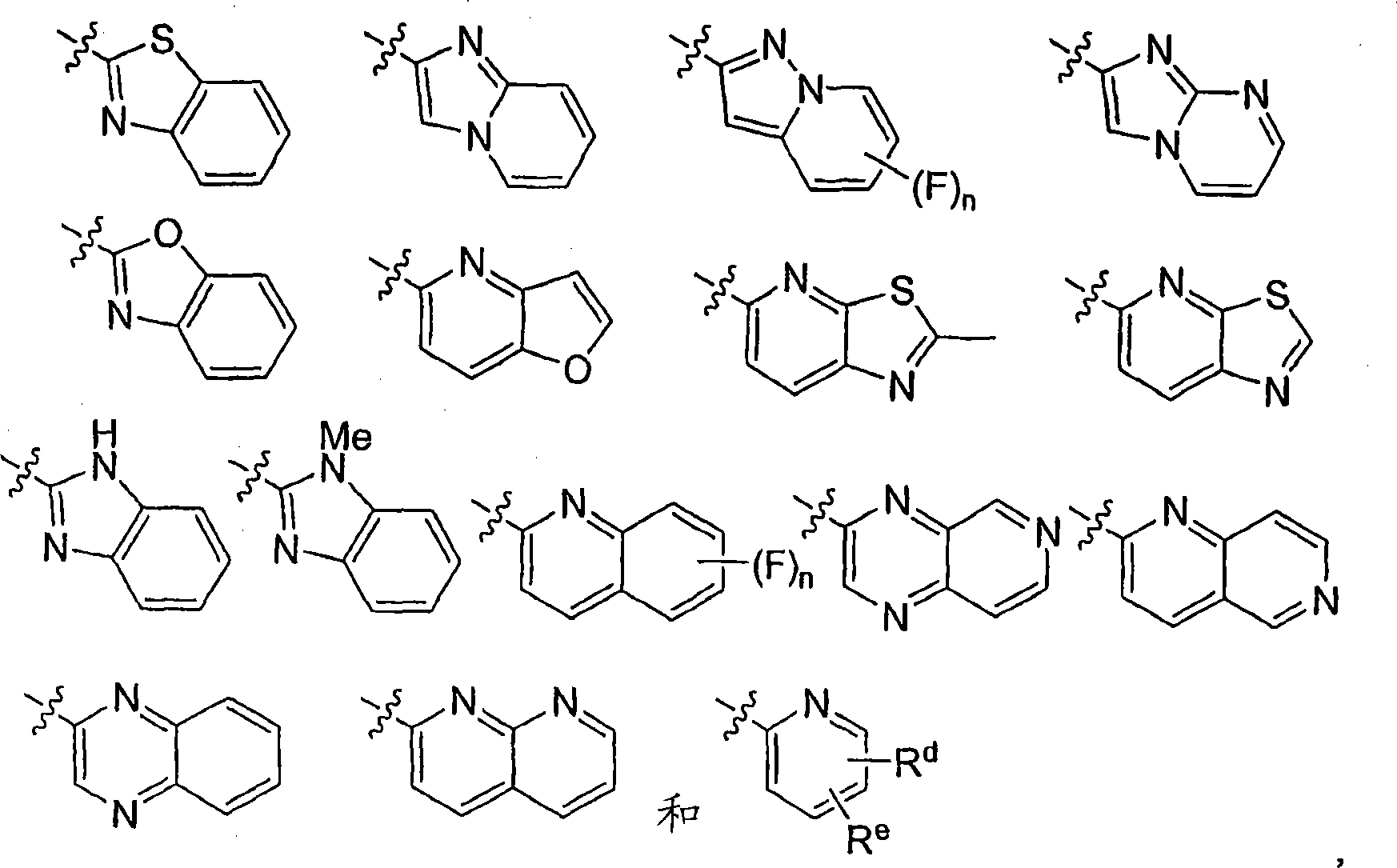
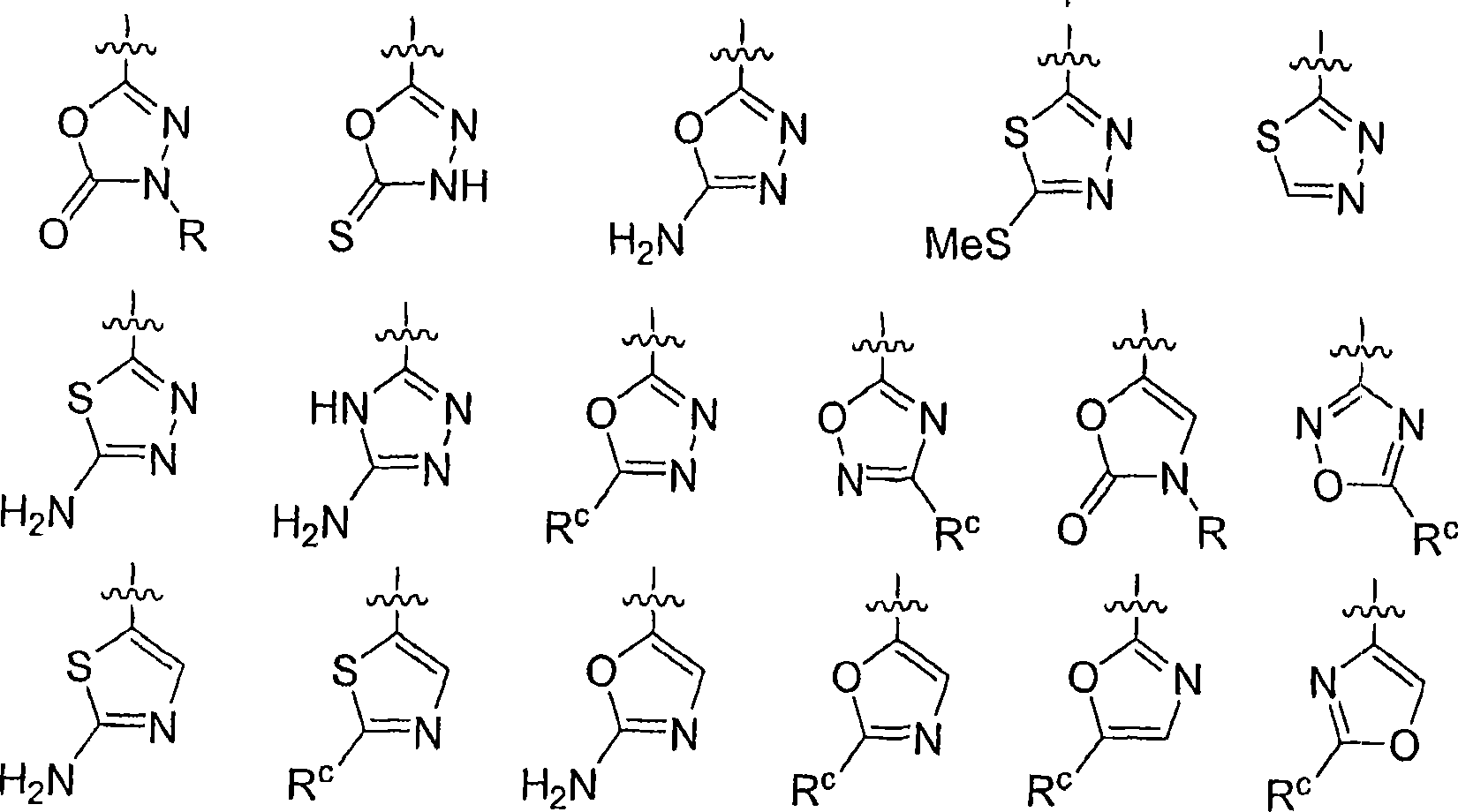
Diphenyl substituted cycloalkanes, compositions containing such compounds and methods of use
A compound, phenyl technology, applied in the field of compounds that inhibit 5-lipoxygenase-activated protein, can solve problems such as danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0297] Reaction scheme 1
[0298]
[0299] Step A: 2,2'-[[2-(1-phenylcyclopentyl)-1,4-phenylene]bis(oxymethylene)]bis -1,3-Benzothiazole (1a) and 4-(1,3-Benzothiazol-2-ylmethoxy) - Preparation of 2-(1-phenylcyclopentyl)phenol (1b)
[0300] At room temperature, 2-(chloromethyl)-1,3-benzothiazole (57.0 mg, 0.31 mmol; according to Mylari, B.L.; Scott, P.J.; Zembrowski, W.J. Synth. prepared), potassium iodide (51.0 mg, 0.31 mmol) and potassium carbonate (71.0 mg, 0.52 mmol) were added to a stirred solution of i-1e (65.0 mg, 0.26 mmol) in DMF (0.75 mL). After 18 hours, the reaction mixture was poured into saturated aqueous ammonium chloride and extracted three times with EtOAc. The combined organic extracts were washed with water, brine, and dried (MgSO 4 ) and concentrated in vacuo. The crude residue was purified by silica gel flash chromatography (gradient elution; 0-30% EtOAc / hexanes as eluent; 4% triethylamine modifier) to afford the title compounds 1a and 1b. ...
Embodiment 2
[0308] Reaction Scheme 2
[0309]
[0310] Step A: 4-[(6-Fluoroquinolin-2-yl)methoxy]-2-(1-phenylcyclobutyl)phenol Preparation of (2a)
[0311] Compound 2a can be prepared from intermediate i-2d and 2-(bromomethyl)-6-fluoroquinoline according to the method outlined in Step A of Scheme 1 .
[0312] Step B: 4-[(6-Fluoroquinolin-2-yl)methoxy]-2-(1-phenylcyclobutyl)phenyl Preparation of triflate (2b)
[0313] Trifluoromethanesulfonic anhydride (1.3 equiv) was added dropwise to a stirred solution of 2a (1.0 equiv) in pyridine / toluene (1:1) at 0°C. The resulting mixture was warmed to room temperature and aged until the reaction was considered complete. The reaction mixture was poured into water and extracted three times with EtOAc. The combined organic extracts were washed with water and dried (MgSO 4 ) and concentrated in vacuo. The crude residue was purified by flash chromatography on silica gel to afford the title compound 2b.
[0314] Step C: 4-[(6-Fluoroqui...
Embodiment 3
[0326] Reaction scheme 3
[0327]
[0328] Step A: 4-[(6-Fluoroquinolin-2-yl)methoxy]-2-(1-phenylcyclopentyl)phenol Preparation of (3a)
[0329] Compound 3a can be prepared from intermediate i-1e and 2-(bromomethyl)-6-fluoroquinoline according to the method outlined in Step A of Scheme 1 .
[0330] Step B: 4-[(6-Fluoroquinolin-2-yl)methoxy]-2-(1-phenylcyclopentyl)phenyl Preparation of triflate (3b)
[0331] Compound 3b can be prepared from intermediate 3a according to the method outlined in Step B of Scheme 2.
[0332] Step C: 2-{[4-allyl-3-(1-phenylcyclopentyl)phenoxy]methyl}-6- Preparation of fluoroquinoline (3c)
[0333] Lithium chloride (5.0 equiv), [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (0.016 equiv) and allyl(tributyl)stannane ( 2.0 equiv) was added to a solution of 3a in 1-methyl-2-pyrrolidone and the resulting mixture was irradiated in a microwave apparatus (300W) at 120°C until the reaction was considered complete. The reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com