Method of manufacturing high purity alpha-terpineol
A high-purity technology of terpineol, which is applied in the field of preparation of high-purity α-terpineol, can solve the problems of products not meeting the requirements of use, difficulties in separation and purification, troublesome catalyst treatment, etc., and achieve easy purchase and industrial production , easy handling and regenerating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of high-purity α-terpineol, comprising the following steps:
[0028]The first step is to synthesize α-terpineol: mix turpentine oil or one of industrial-grade α-pinene or β-pinene, an organic solvent, and water, and mix them with sulfuric acid, phosphoric acid, p-toluenesulfonic acid, and chloroacetic acid. One or two or more mixtures or macroporous cation exchange resins are used as catalysts to generate α-terpineol reaction products in direct hydration reactions. In this process, by controlling turpentine or industrial-grade α-pinene or β- One of the pinene, organic solvent, water ratio and reaction temperature form a transparent homogeneous solution system method to reduce the generation of γ-terpineol, as long as the reactants form a transparent homogeneous solution system, it can effectively inhibit The generation of gamma-terpineol, thereby effectively increases the purity of alpha-terpineol, wherein the quality of catalyst is 1%~20% of turpen...
Embodiment 2
[0038] A preparation method of high-purity α-terpineol, comprising the following steps:
[0039] Step 1: The present invention uses an organic solvent as a reaction medium, water as a reaction reagent, and a macroporous cation exchange resin as a catalyst to generate α-terpineol by direct hydration in a homogeneous solution system. Raw materials can use α-pinene, β-pinene, or turpentine oil with α-pinene and β-pinene as the main components. The turpentine oil can be gum turpentine, sulfate turpentine, wood turpentine; the organic solvent can be C1~C6 Low-carbon fatty alcohols such as ethanol, isopropanol, etc., C1-C6 low-carbon fatty acids (such as acetic acid) and their esters (such as ethyl acetate), etc.; the reagent water can be distilled water, or tap water, etc., no need to carry out Special treatment; within a certain proportion range, turpentine, water, organic solvents, etc. can form a stable and uniform solution system, especially within the reaction temperature rang...
Embodiment 3
[0045] Add calculated amount of turpentine 68g (0.5mol), distilled water 11g (0.61mol) and solvent isopropanol 110g in a round bottom flask equipped with agitator, reflux condenser, and thermometer, and form a transparent homogeneous solution after stirring. All should keep homogeneous solution state in non-heating, heating and reaction process; Add catalyst D-72 macroporous cationic resin 4g (accounting for 5% of raw material quality). Heat the mixture to 90°C, adjust the heating rate to keep the reaction solution at 89-91°C, and react for about 12 hours. During the reaction, samples can be taken for follow-up analysis, and the reaction is generally stopped when the mass fraction of pinene reaches about 8%.
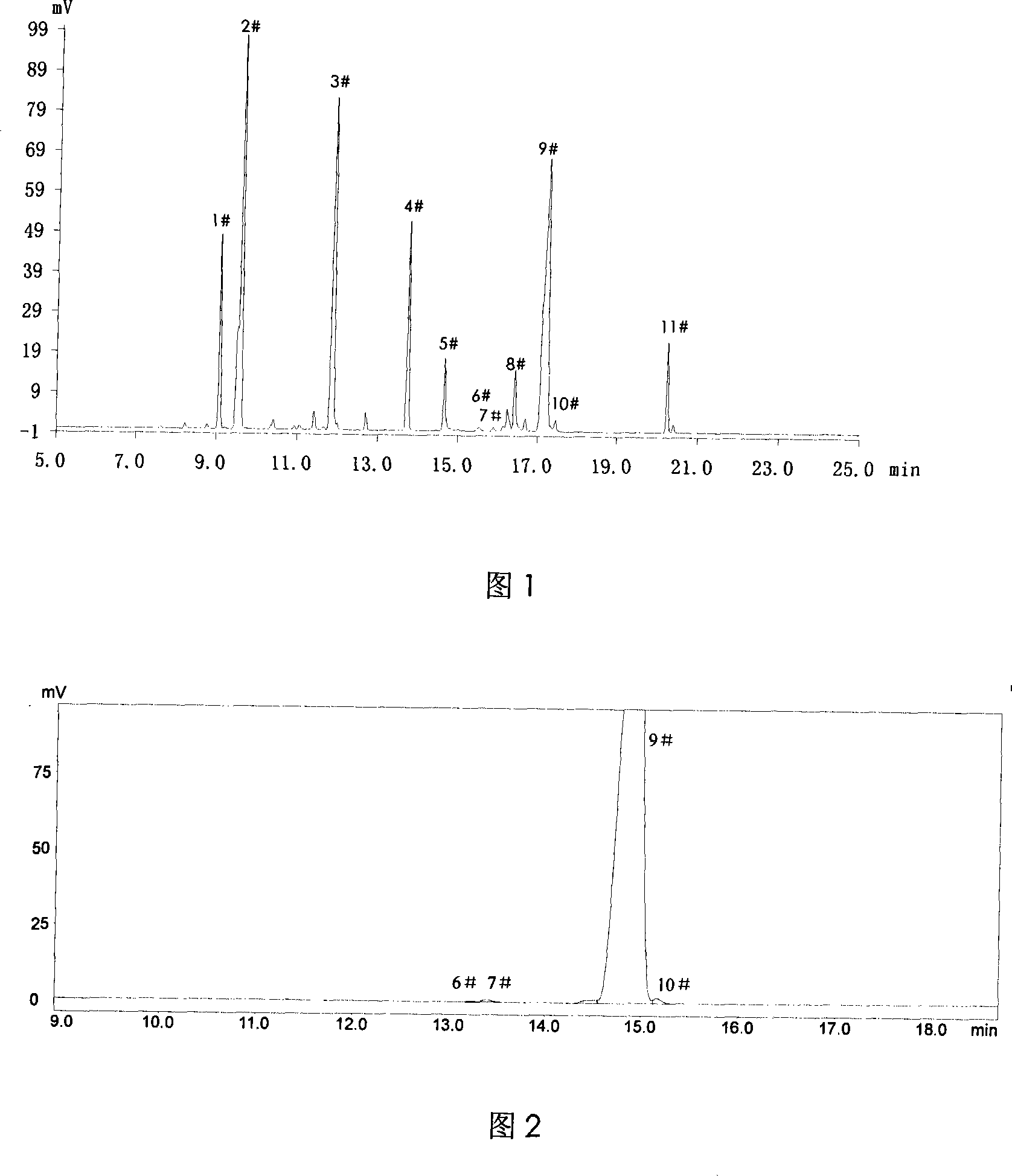
[0046] The mass fractions of main components in the reaction solution obtained after post-treatment are: 35.7% of α-terpineol and 0.9% of γ-terpineol (see accompanying drawing 1 for an example of GC analysis spectrum).
[0047] The mass fraction of the main component α-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com