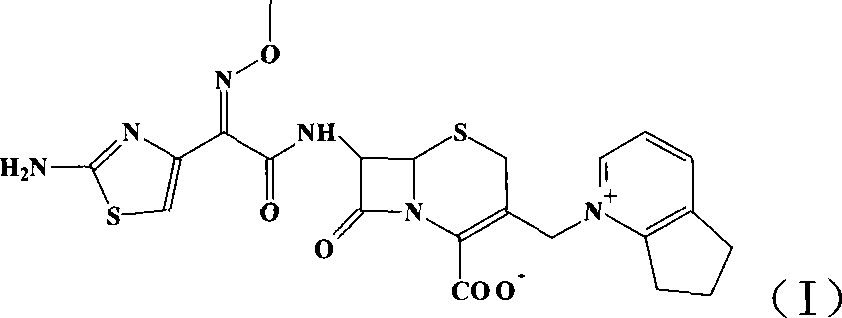
Technique for preparing high-purity cefpirome sulfate
A technology of cefpirome sulfate and organic solvents, which is applied in the field of refining cefpirome sulfate, can solve the problems of high energy consumption, etc., and achieve the effects of short production time, large output, and good industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 300g of crude cefpirome sulfate (0.49mol, the weight is based on anhydrous matter) to 1500mL of water at room temperature (15-25°C), and add about 20% sodium hydroxide aqueous solution dropwise under vigorous stirring until the pH of the system is 6.5-7.0 between. Then add 3000mL acetone and appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with 800 mL of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. Add 30% H to the filtrate with stirring 2 SO 4 to pH 1.0-1.5. Then 9 L of acetone was added. After the crystals were precipitated, the temperature of the system was lowered to 5-10°C, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 270 g of white crystalline powder. The purity is above 99%, and the water content is 3-4%. The product is cefpirome sulfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com