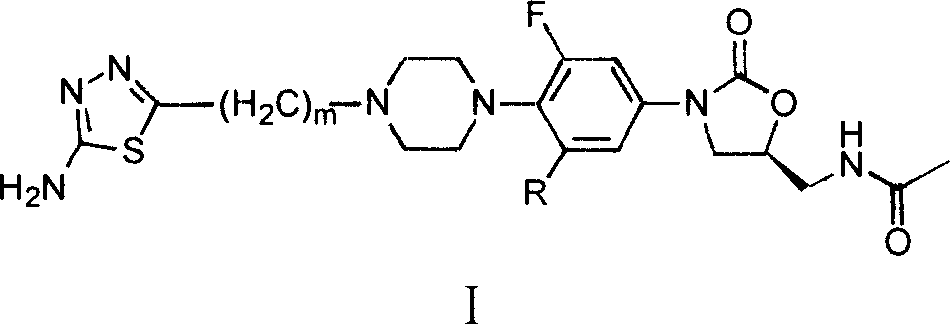
Oxazolidinone compound containing thiadiazoles groups and preparation method thereof
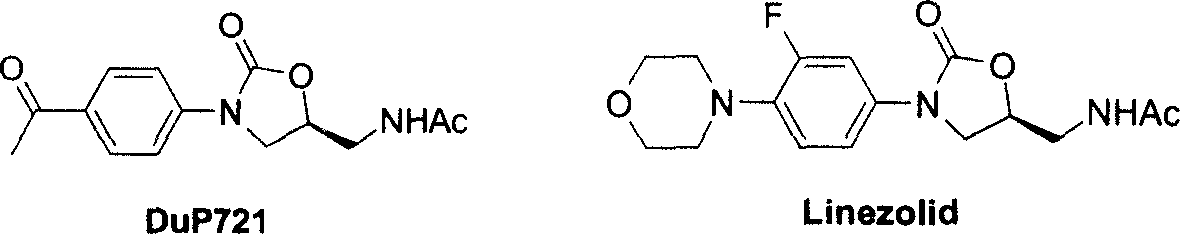
A compound, thiadiazole technology, applied in the application field of oxazolidinone compounds and/or hydrates to prepare and treat microorganisms, especially bacterial infectious diseases, can solve ineffective, such as methicillin-resistant Staphylococcus aureus , Methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus, reduced efficacy of antibacterial drugs, difficult treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 (S)-N-[[3-[3-fluoro-4-[4-(5-amino-[1,3,4]thiadiazol-2-ylmethyl)-1-piperazine Preparation of base]phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide
[0052] [1] Preparation of 1-(2-fluoro-4-nitrophenyl)piperazine
[0053] Dissolve 25.8g (0.3mol) of anhydrous piperazine in 250ml of acetonitrile, stir and raise the temperature to 40°C, slowly add 11.4ml (0.1mol) of 3,4-difluoronitrobenzene dropwise, heat to reflux for 3h, and cool , evaporated to dryness under reduced pressure, 100 ml of water was added to the residue, extracted with ethyl acetate, the organic layers were combined, washed with water, dried, and evaporated to dryness under reduced pressure to obtain 18.9 g of yellow solid (yield 84%).
[0054] [2] Preparation of N-benzyloxycarbonyl-3-fluoro-4-(N-benzyloxycarbonyl-1-piperazinyl)aniline
[0055] Dissolve 18.9g (0.084mol) of 1-(2-fluoro-4-nitrophenyl)piperazine in 200ml of tetrahydrofuran, add 1.8g of 10% palladium carbon, hydrogenate and reduce under ...
Embodiment 2
[0072] Example 2 (S)-N-[[3-[3-fluoro-4-[4-(5-amino-[1,3,4]thiadiazol-2-ylethyl)-1-piperazine Base]phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide hydrochloride
[0073]
[0074](S)-N-[[3-[3-fluoro-4-[4-cyanoethyl-1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl ] Add 3.9g (0.01mol) of acetamide to 10ml of toluene, add 4ml of concentrated hydrochloric acid to the reaction solution, stir, add 0.9g (0.01mol) of thiosemicarbazide, and heat to reflux for 5h. After the reaction is complete, cool, adjust the pH to 8 with saturated sodium carbonate solution at 0-5°C, extract with toluene, combine the organic layers, wash with water, dry, evaporate to dryness, add the obtained solid to acetone, and adjust the pH to 1- 2. Heating to reflux for 0.5h, cooling, standing overnight, crystallization, suction filtration, drying to obtain (S)-N-[[3-[3-fluoro-4-[4-(5-amino-[1, 3,4] Thiadiazol-2-ylmethyl)-1-piperazinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide hydrochloride 4.2g, yield w...
Embodiment 3-6
[0076] Example 3-6 compound
[0077] Table 2 is the compound prepared by embodiment 3-6
[0078] Example
Chemical Name
yield
[0079] The preparation of the tablet of embodiment 7 embodiment 1 compound
[0080] Get Example 1 compound 250g, starch 30g, 2% HPMC aqueous solution 80ml, carboxymethyl starch sodium 15g, magnesium stearate 2g, follow the steps below:
[0081] a. Prepare an appropriate amount of 2% HPMC solution and set aside;
[0082] b. Properly dry the raw materials and auxiliary materials, pass through a 100-mesh sieve respectively, and set aside;
[0083] c. Weigh the raw and auxiliary materials according to the prescription quantity. After mixing the compound of Example 1, starch, and sodium starch glycolate evenly, add 2% HPMC solution to make soft material, and use a 20-mesh sieve to make wet granules;
[0084] d. Dry the wet granules at 55°C for about 3 hours, let cool slightly after drying, add magnesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com