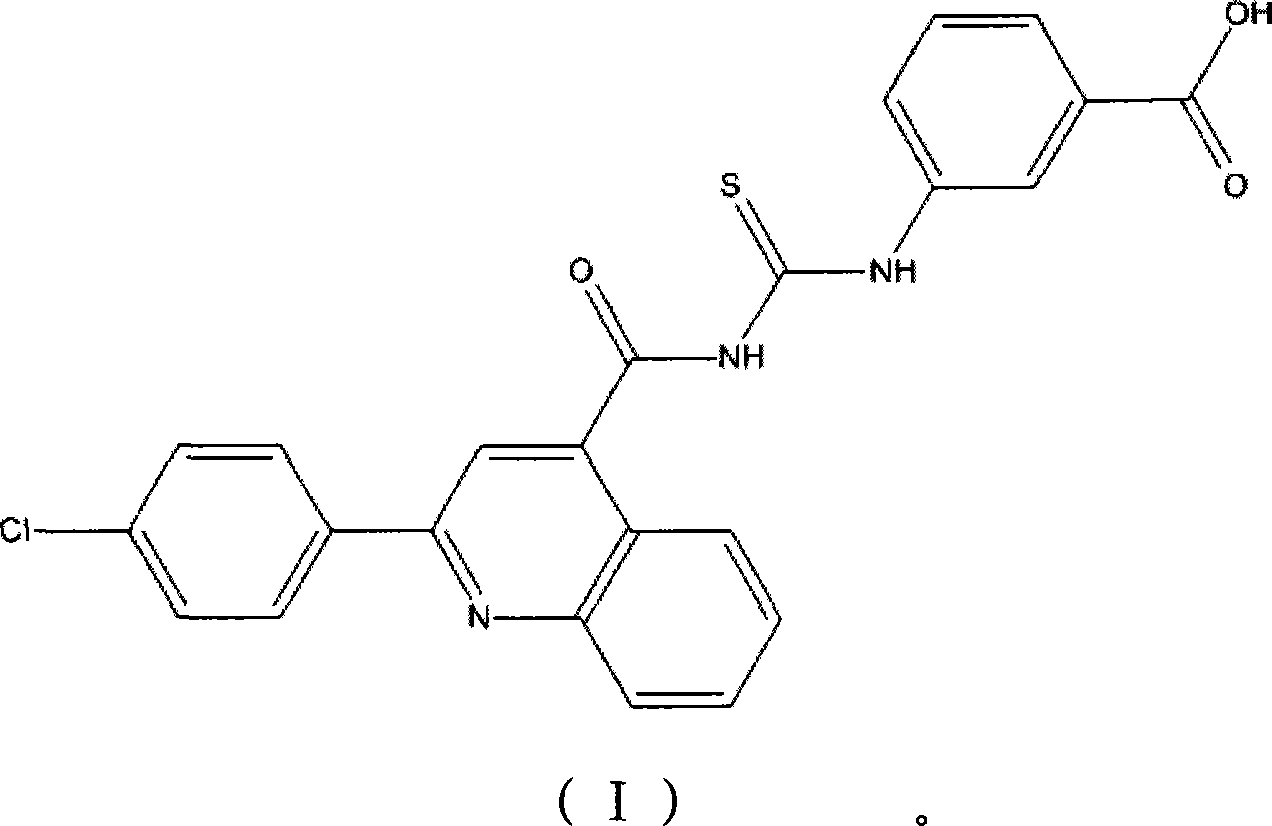
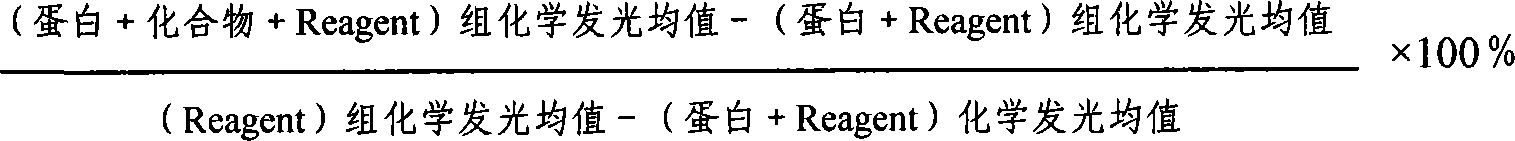Staphylococcus tryptophanyl-tRNA synthetase inhibitor
A technology of staphylococcus and staphylococcus epidermidis, applied in the direction of active ingredients of heterocyclic compounds, antibacterial drugs, etc., can solve the problem of severe infection and easy development of chronic infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Construction of tryptophanyl-tRNA synthetase prokaryotic expression plasmid
[0015] (1) Construction of the prokaryotic expression plasmid of Staphylococcus epidermidis tryptophanyl-tRNA synthetase
[0016] Staphylococcus epidermidis tryptophanyl-tRNA synthetase gene (NC_004461, locus tag: SE0685) acquisition method is as follows:
[0017] Design an Nco I restriction site at the 5' end of the sense strand primer, and an Nhe I restriction site at the 5' end of the antisense strand primer:
[0018] sense:
[0019] 5'-CC CCA TGG AAA CTT TAT TCT CAG GA-3’
[0020] anti-sense:
[0021] 5’-CG GCT AGC TTA TCT TTT TCG ACC TAA GCC C-3’
[0022] The underlined sequence is the restriction site of Nco I, and the sequence in italics is the restriction site of Nhe I.
[0023] The coding gene of Staphylococcus epidermidis tryptophanyl-tRNA synthetase was amplified by PCR using the above primers. Digestion by restriction endonucleases (NcoI and NheI) and T 4 DNA lig...
Embodiment 2
[0031] Example 2 Expression and purification of tryptophanyl-tRNA synthetase protein
[0032] Host bacteria: Escherichia coli BL21-DE3
[0033] 1) Pick a single clone colony and inoculate into 5ml LB medium, 37℃×250rpm to OD 600 =1~2.
[0034] 2) Inoculate 10ul to 5ml LBK medium, add 1M IPTG to a final concentration of 1mM, and induce expression for 4h.
[0035] 3) The bacterial cells were collected by centrifugation, resuspended in PBS, ultrasonically disrupted, centrifuged at 12,000 rpm for 5 minutes at 4°C, and the supernatant was obtained. with Invitrogen ProBond TM Purification System purifies protein. The distribution of the target protein was determined by 12% SDS-PAGE protein electrophoresis analysis. Proteins were quantified by the Bradford method.
Embodiment 3
[0036] The influence of embodiment 3 compound FDDDC02 on tryptophanyl-tRNA synthetase activity:
[0037] 1. The effect of compound FDDDC02 on the activity of staphylococcal tryptophanyl-tRNA synthetase and the activity of human tryptophanyl-tRNA synthetase (using two methods for detection):
[0038] 1) Detection of the reduction of the substrate ATP - Kinase-Glo TM Luminescent Kinase Assay Kit (Promega, USA): 2 μg recombinantly expressed and purified tryptophanyl-tRNA synthetase from Staphylococcus epidermidis, 2 μg tryptophanyl-tRNA synthetase from Staphylococcus aureus and 3 μg human tryptophanyl-tRNA synthetase Respectively react with the compound FDDDC02 of gradient dilution at 25°C for 20 minutes, then add 100 μM ATP (total reaction volume 50 μL), and act at 25°C for 20 minutes, add the reaction mixture to a 96-well microtiter plate, 50 μL per well, add Kinase- Glo TM 50 μL of Reagent per well was allowed to stand at room temperature for 10 minutes, and the chemilumin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com