Therapeutic compounds comprised of anti-fc receptor binding agents
A receptor and antibody technology, applied in antibody medical components, antibody mimics/scaffolds, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0085] example 1 : Production of bispecific antibodies comprising murine or humanized antibodies specific for Fc receptors and anti-her2 neu antibodies
[0086] Monoclonal antibodies
[0087] Anti-FcγRI monoclonal antibodies (mAbs), M22, M32.2, and 197 were purified from hybridoma supernatants by ion-exchange chromatography, and from hybridomas by protein A affinity chromatography (Pharmacia, Piscataway, NJ) and gel filtration. DZ33, a human anti-HIV-1 IgGl mAb, was purified from tumor supernatants. M32.2 was deposited with the American Type Culture Collection (12301 Parklawn Drive, Rockville, MD20852) on July 1, 1987, and the determined ATCC accession number is HB9469.
[0088] cell line
[0089] Murine myeloma NSO (ECACC85110503) is a non-Ig synthetic cell line that was used to express recombinant mAbs. NSO cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Gibco, Paisley, U.K.). SKBR-3 is a human breast cancer cell line that overexpresses t...
example 2
[0136] Example 2 : Generation of functional H22-epidermal growth factor fusion protein
[0137] Materials and methods
[0138] Expression Vectors and Cloning
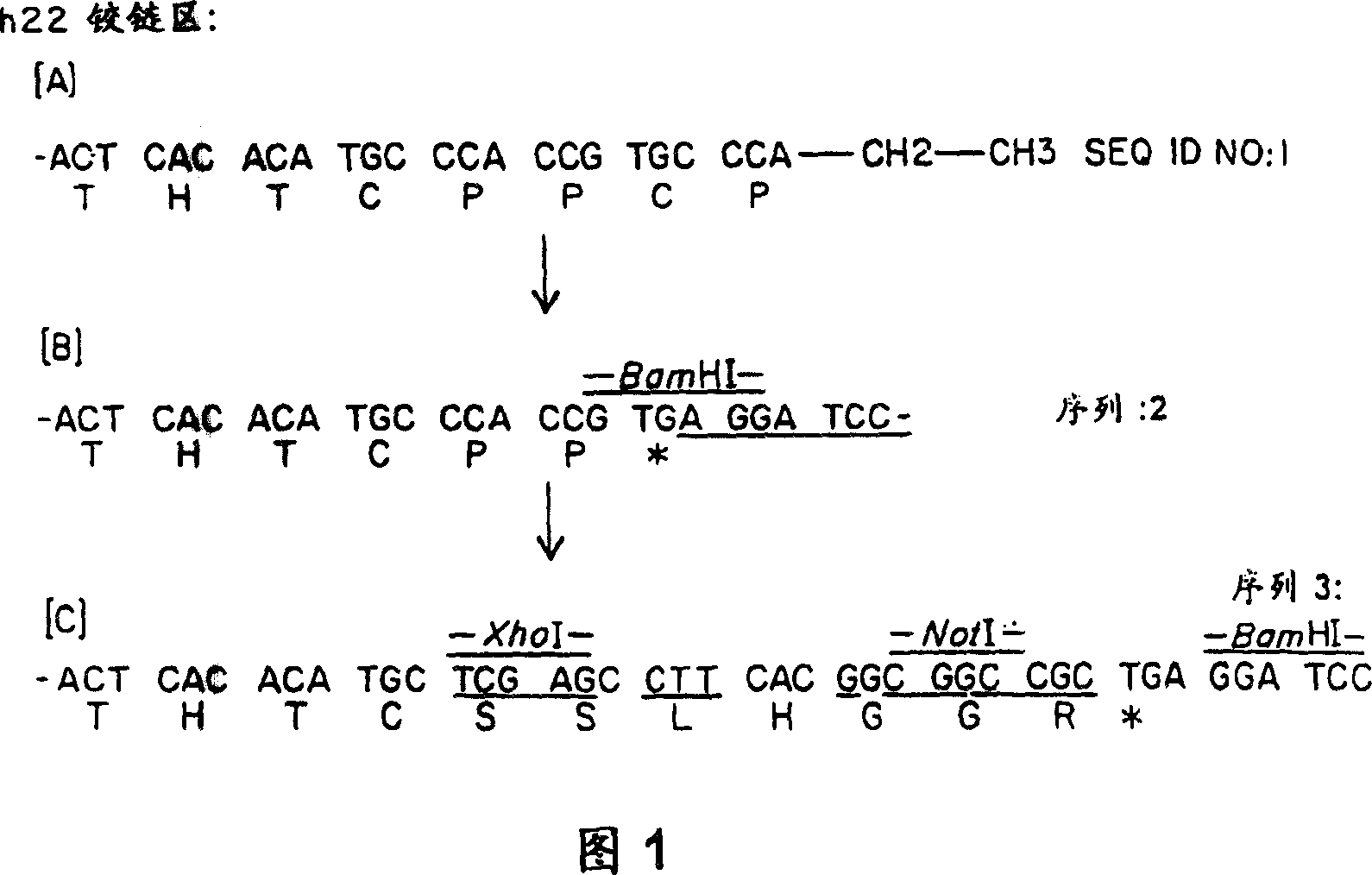
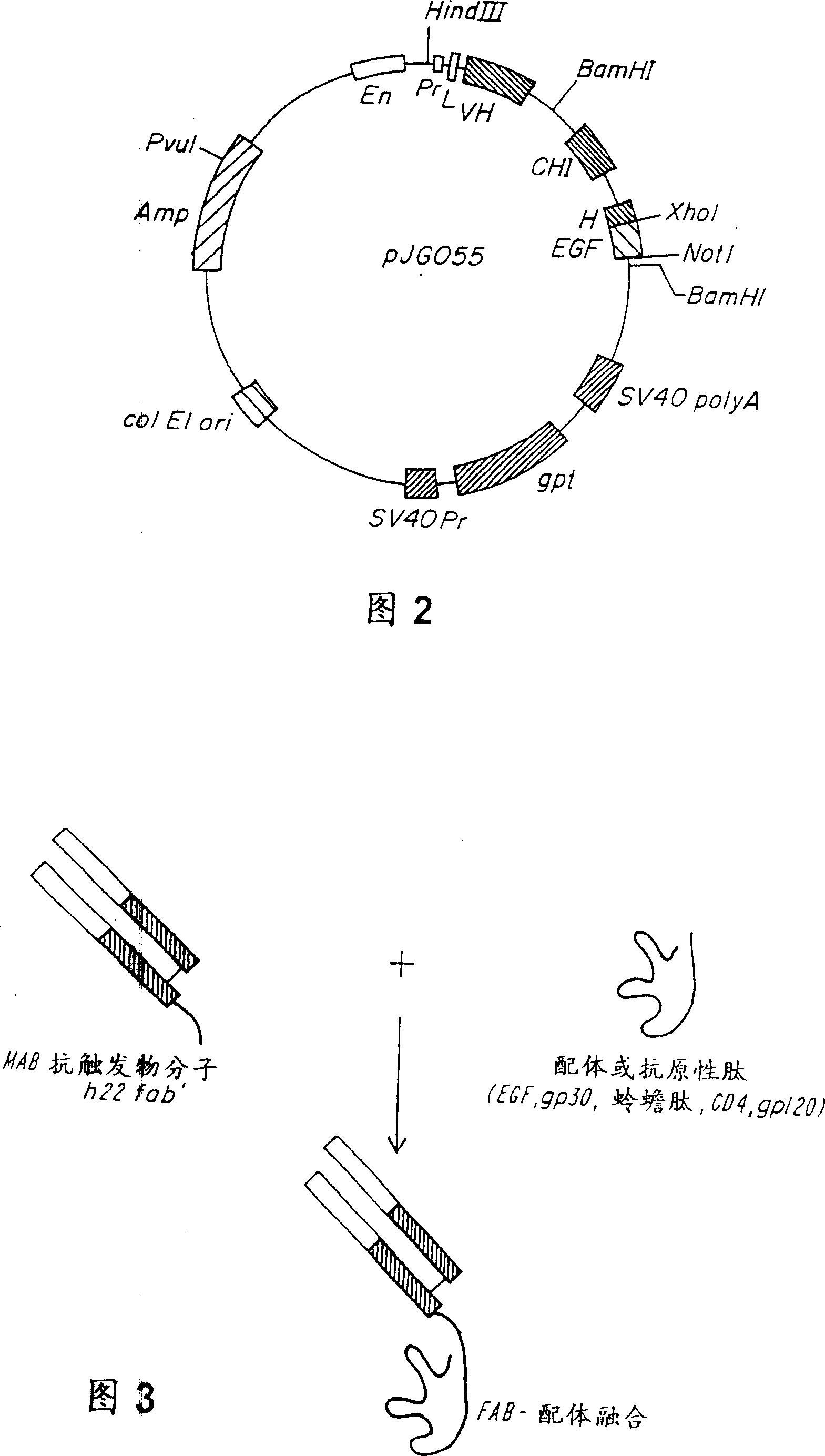
[0139]Expression vectors (pSVgpt and pSVhygt) for genomic cloning of the heavy and light chains of H22 are disclosed in WO 94 / 10332 entitled "Humanized Antibodies to FcReceptors for Immunoglobulin G on Human Mononuclear Phagocytes". For Fab-ligand fusion constructs, no changes to the light chain are required. However, for the heavy chain, it is necessary to remove the CH2 and CH3 regions and replace them with the coding sequence for the ligand. The heavy chain vector carries two BamHI sites, one in the intron between VH and CH1 and the other downstream of CH3. Using the BamHI restriction site described above, the DNA encoding the constant region was replaced with a truncated version encoding only CH1 and most of the hinge region. To this end, the polymerase chain reaction (PCR) was used to generate a new C-ter...
example 3
[0163] Example 3: H22-Heregulin (H22-gp30) fusion protein mediates tumor cell death
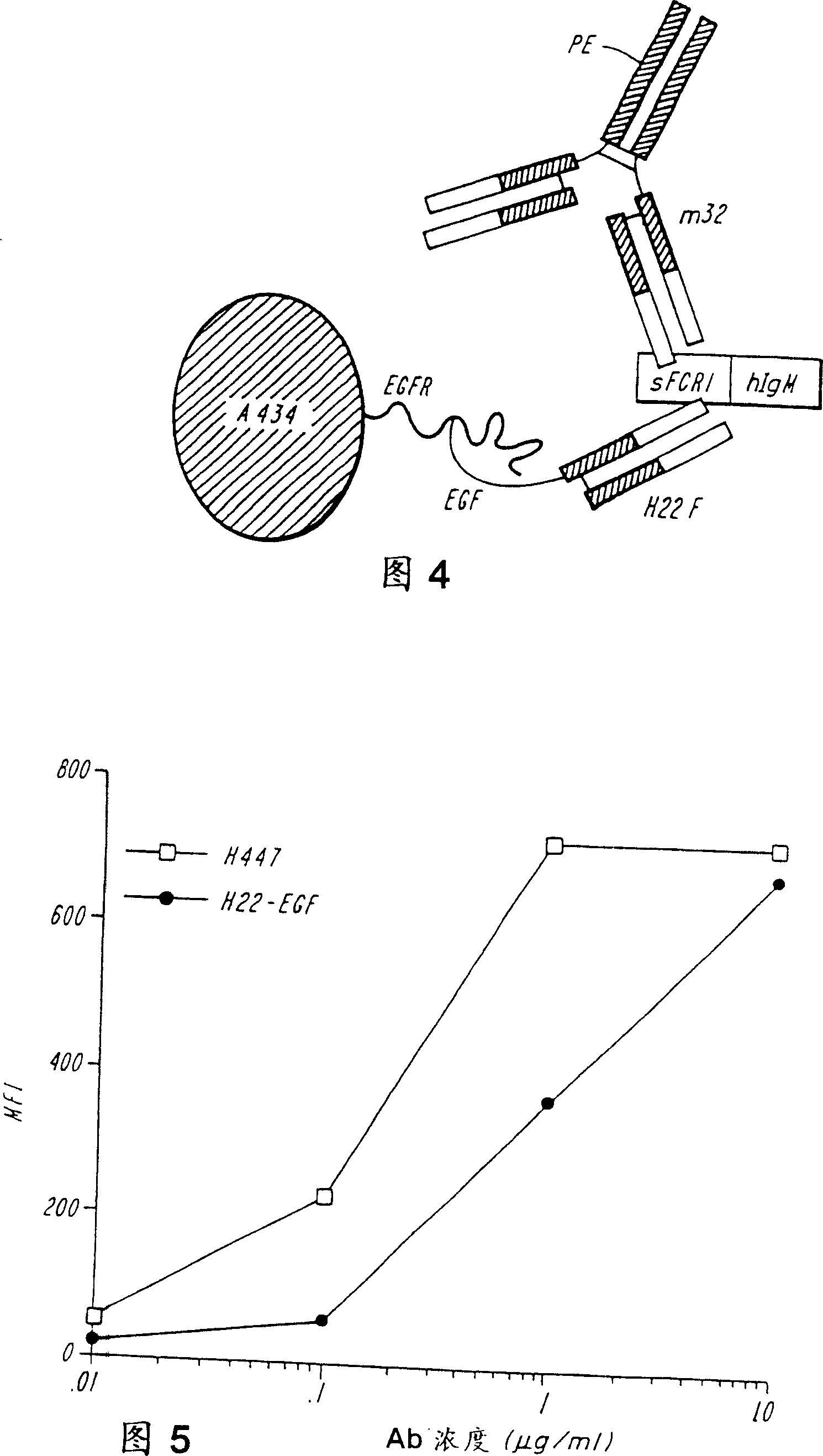
[0164]Heregulin (HRG) is a ligand for HER3 and HER4 molecules. Both receptors form heterodimers with HER2, a molecule overexpressed by certain breast cancer cells. The affinity of HRG for HER3 and HER4 is significantly increased when the molecule is derived from a heterodimer with HER2. This example demonstrates that bispecific molecules comprising heregulin and a binding specificity for FcγRI inhibit the growth of tumor cell lines and mediate fusion protein-dependent fusion of these cells in the presence of FcγRI-bearing cytotoxic effector cells. sex cytotoxicity.
[0165] The H22-heregulin fusion protein was constructed in the same manner as the H22-EGF fusion protein described in Example 2. Briefly, the genomic DNA encoding the Fd fragment of the humanized anti-FcyRI mAb, H22, was fused to the cDNA encoding the EGF region of β2-type HRG. The amino acid sequence of the H22-HRG fusion pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com