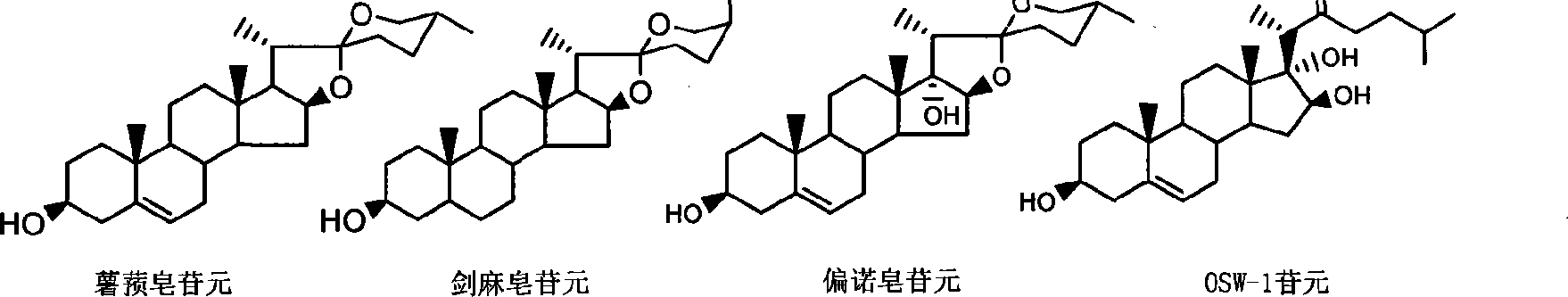
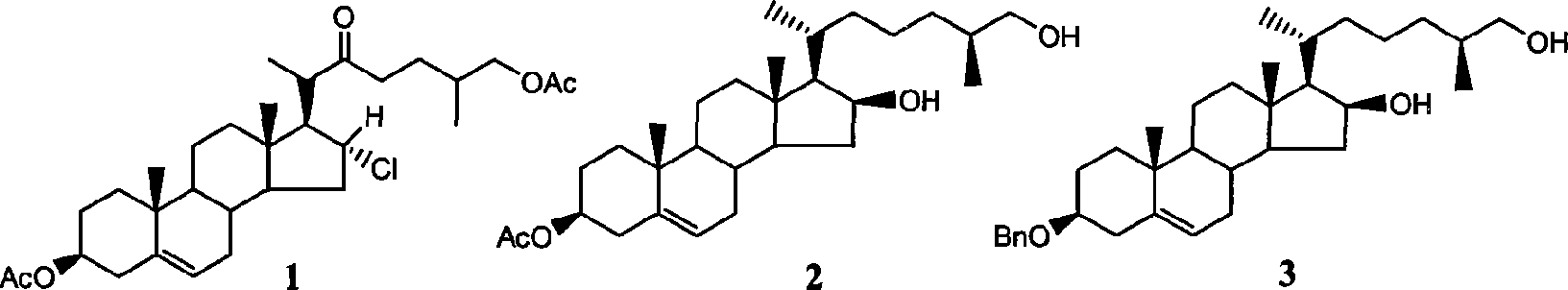
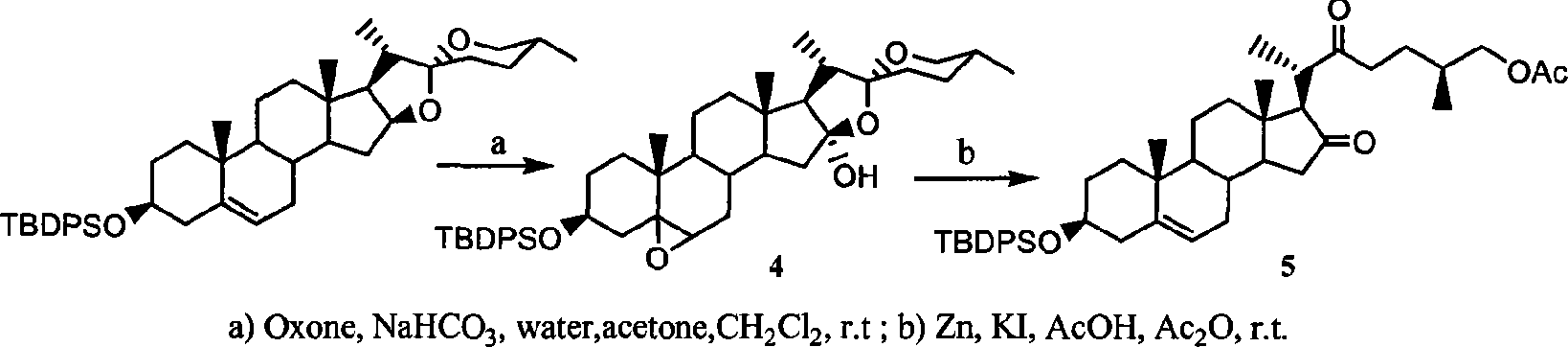
Complete E/F ring-opening synthesis process of 26-chloro-3beta, 16beta-diacetyloxy-22-one-(5-) cholestane (cholestene) with sterioside
A technology of diacetoxy and steroidal saponins, applied in steroids, organic chemistry and other directions, can solve problems such as low yield, and achieve the effects of high atom utilization and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] 1: Acetylation of Diosgenin and Sisalogenin
[0031] General method:
[0032] Put 1 mol of diosgenin (sisalgenin) in a 1000mL reaction bottle, add ethyl acetate with a mass of about 3 to 5 times as a solvent and about 5% mol of diosgenin (sisalin) as a catalyst , stir and slowly add 1.1 mol of acetic anhydride as esterification agent at room temperature, and then reflux for about 8-9 hours after the addition of acetic anhydride is completed. After the reaction is detected by TLC, pour the reaction mixture into another container and add an appropriate amount of NaHCO 3 Saturated solution, adjust the pH value to about 6-7, separate the ethyl acetate phase, extract the water phase twice with an appropriate amount of ethyl acetate, combine the organic phases, wash the organic phase once with saturated saline, and dry over anhydrous sodium sulfate. The solid residue obtained after stripping the solvent was purified by recrystallization from methanol.
[0033] Acetyldiosge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com