Magnetic target medicine sustained and controlled release carrier material and preparation method and application thereof
A controlled-release carrier and magnetic targeting technology, which is applied in drug delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of weak magnetic response, low orientation efficiency, drug loading and encapsulation efficiency of single magnetic particles. Incompletely controllable and other issues, to achieve the effect of high magnetic response strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] step 1)
[0041] Get 2ml of polystyrene (PS) emulsion (average particle diameter 230nm) with a concentration of 80mg / ml and disperse it in 250ml deionized water, add FeCl 2 aqueous solution, Fe in the system 2+ The concentration is 0.02mol / L, after ultrasonic dispersion for several minutes, add 0.0015mol hexamethylenetetramine (HMTA) under stirring, pH=11, raise the temperature to 70~80℃ for 3h, then separate the product with a magnetic field, use Wash the product with ionic water for 3 to 5 times, dry the product at 60°C for 24 hours, and the product is Fe 3 o 4 Nanoparticle-coated magnetic composite spheres of polymer microspheres.
[0042] Step 2)
[0043] The product obtained in step 1) was placed in a muffle furnace, protected by nitrogen, and slowly heated to 500° C. at a heating rate of 10° C. / min. It was calcined for 3 hours and then taken out.
[0044] step 3)
[0045] A polyelectrolyte solution was prepared by dissolving poly(allylamine hydrochloride, PA...
Embodiment 2
[0052] Replace the polystyrene microspheres in embodiment 1 step 1) with 800nm polystyrene microspheres, replace the solution A in embodiment 1 step 3) with the acetic acid solution of 10mg / ml chitosan, use 10mg / ml PAA aqueous solution Instead of solution B in step 3) of Example 1, the deionized water in step 3) of Example 1 was replaced with acetic acid / sodium acetate standard buffer solution with pH=3.6, and steps 1-4 of Example 1 were repeated for others. Finally, magnetic composite hollow spheres coated with 4 double-layer polyelectrolyte multilayer films were obtained.
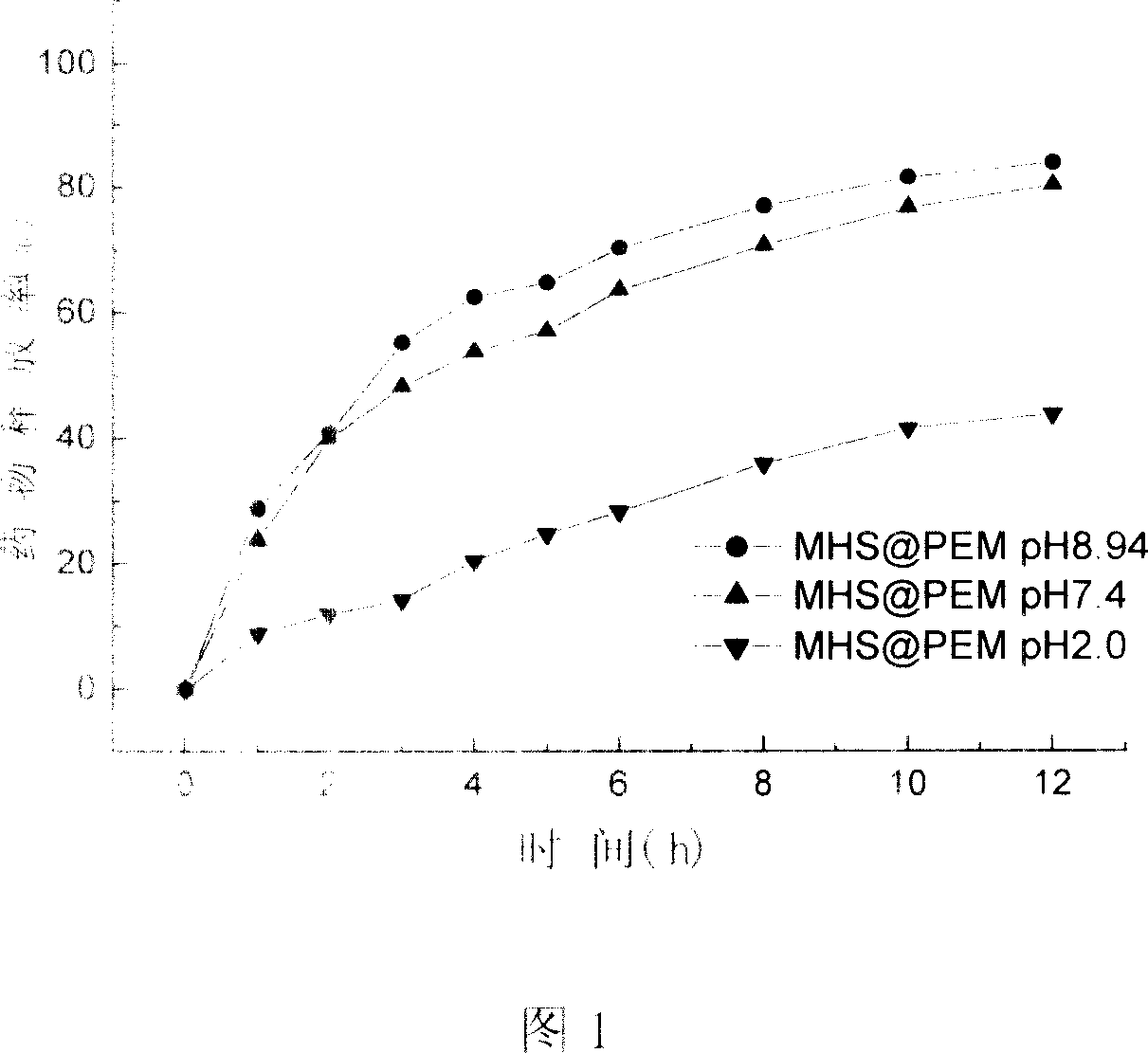
[0053] The drug release performance evaluation method is the same as that in Example 1, and the results show that the drug release rate increases with the increase of the pH value of the system, and the release rate in the pH=9.0 medium is close to twice that in the pH=2.0 medium.
Embodiment 3
[0055] Replace the polystyrene microsphere in embodiment 1 step 1) with the polymethyl methacrylate microsphere of average diameter 400nm, use Zn(Ac) 2 and FeCl 2 The mixed solution replaces embodiment 1 step 1) FeCl 2 solution, Zn in the system 2+ The concentration is 0.01mol / L, Fe 2+ with Zn 2+ The molar ratio is 2: 1, replace the hexamethylenetetramine in embodiment 1 step 1) with NaOH, system pH=13, other reaction conditions are the same as embodiment step 1), gained product is Zn ferrite nanoparticle A magnetic composite ball coated with polymer microspheres; then repeat steps 2 to 4 in Example 1). Finally, magnetic composite hollow spheres coated with 6 double-layer polyelectrolyte multilayer films were obtained.
[0056] The drug release performance evaluation method is the same as in Example 1, and the results show that in release media of different pH values, the system has shown a sustained release behavior to drug molecules, and the release rate of the drug var...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com