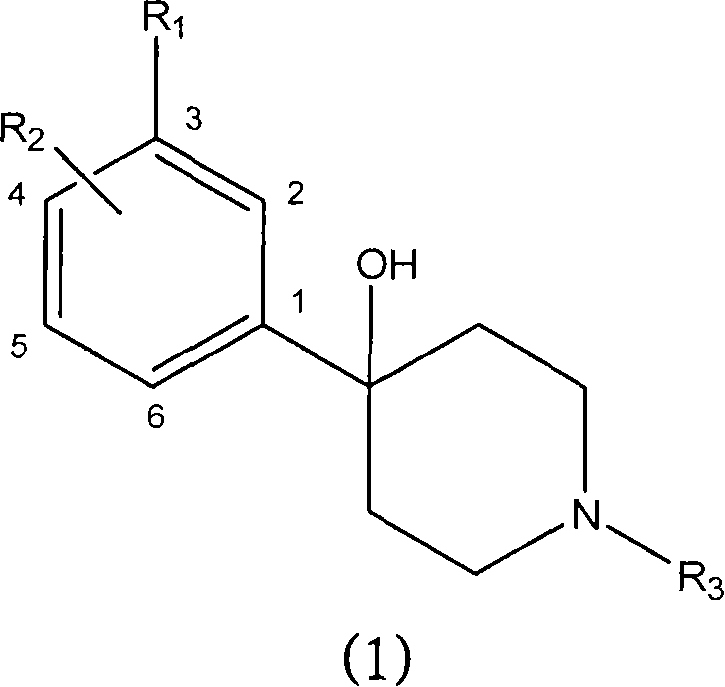
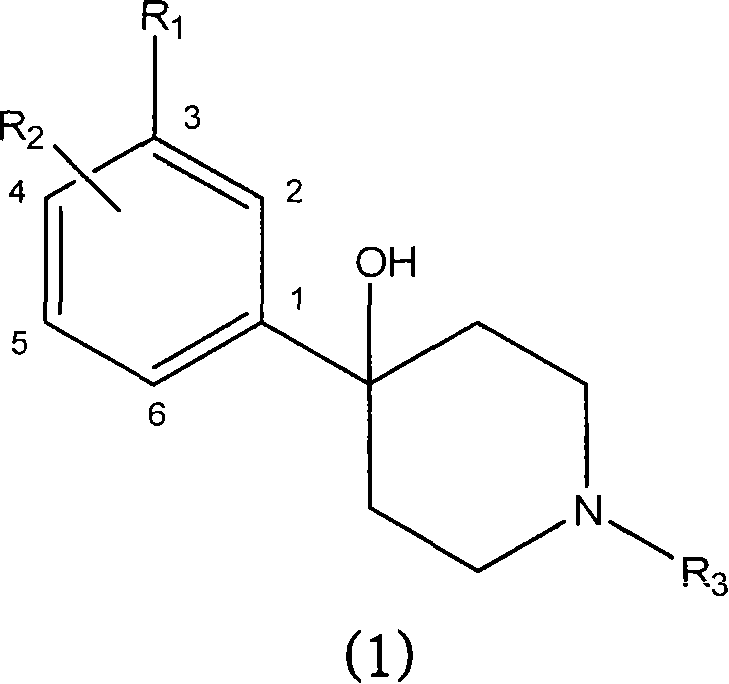
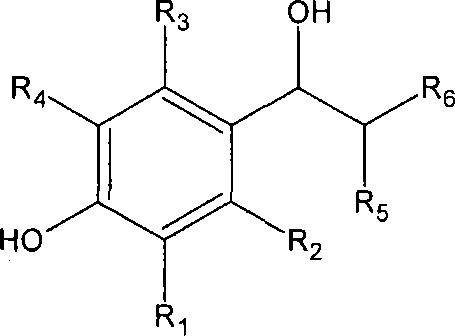
Substituted piperidines as modulators of dopamine neurotransmission
A kind of technology of propylpiperidine and ethylpiperidine, applied in the field of new regulators of dopamine neurotransmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0237] 4-[2-Fluoro-3-(trifluoromethyl)phenyl]-1-propylpiperidin-4-ol
[0238] To a solution of 3-bromo-2-fluorobenzotrifluoride (5.0 g, 20.5 mmol) in dry tetrahydrofuran (70 ml) under nitrogen at -78°C was added n-butyllithium (in hexane 2.5M, 9.0ml, 22.5mmol). The mixture was stirred for 1 hour, after which time a solution of freshly distilled 4-propyl-1-piperidone (2.6 g, 20.5 mmol) in dry tetrahydrofuran (30 mL) was added dropwise. The compound was stirred at -78°C for 30 minutes and then allowed to reach ambient temperature. Water (100ml) was added and the mixture was extracted with ethyl acetate (3x100ml). The combined organic phases were dried (MgSO 4 ), filtered and evaporated to dryness. The oily residue was purified by flash chromatography (ethyl acetate / methanol, 1:1) to give the title compound (2.8 g, 45%). The amine was converted to the hydrochloride and recrystallized from ethanol / ether: M.p. 175-177°C. MS m / z (relative intensity, 70 eV) 305 (M+, 5), 276 (bp...
Embodiment 2
[0240] 4-[4-Chloro-3-(trifluoromethyl)phenyl]-1-(2-methoxyethyl)piperidin-4-ol
[0241] To a mixture of 4-[chloro-3-(trifluoromethyl)phenyl]piperidin-4-ol (0.5g, 1.79mmol) and potassium carbonate (0.62g, 4.47mmol) in acetonitrile (40ml) was added 1-bromo-2-methoxyethane (0.17ml, 1.79mmol) and a small amount of sodium iodide crystals, and the mixture was heated under reflux for 15 hours. The mixture was cooled to ambient temperature, water (50ml) was added and the phases were separated. The aqueous phase was extracted with ethyl acetate (2×50 ml) and the combined organic phases were dried (MgSO 4 ) and evaporated under reduced pressure to give an oil. Purification by flash chromatography (ethyl acetate / methanol, 1:1) afforded the title compound (0.41 g, 70%). The amine was converted to the hydrochloride and recrystallized from ethanol / ether: M.p. 181-183°C. MS m / z (relative intensity, 70 eV) 337 (M+, 1), 294 (29), 292 (bp), 274 (72) 201 (29).
Embodiment 3
[0243] 4-[2-Fluoro-3-(trifluoromethyl)phenyl]-1-ethylpiperidin-4-ol
[0244] Prepared as in Example 1: 3-bromo-2-fluorobenzotrifluoride (5.0 g, 20.6 mmol), tetrahydrofuran (50 ml), n-butyllithium (2.5M in hexane, 9.0 ml, 22.5 mmol), 4 - Ethyl-1-piperidone (2.6 g, 20.6 mmol). Yield: 4.0 g. Conversion of amine to hydrochloride and recrystallization from ethanol / ether: M.p. 177-180°C. MS m / z (relative intensity, 70 eV) 291 (M+, 18), 277 (15), 276 (bp), 258 (37), 191 (27).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com