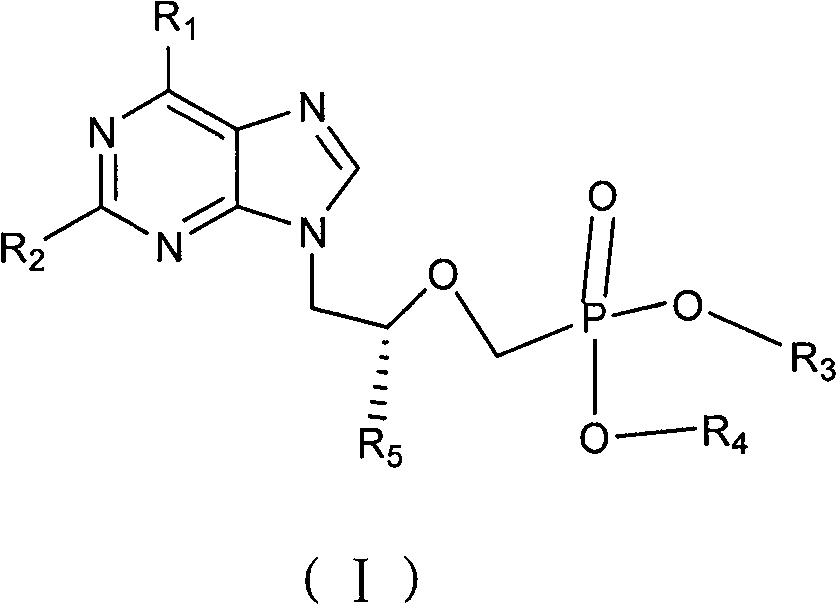
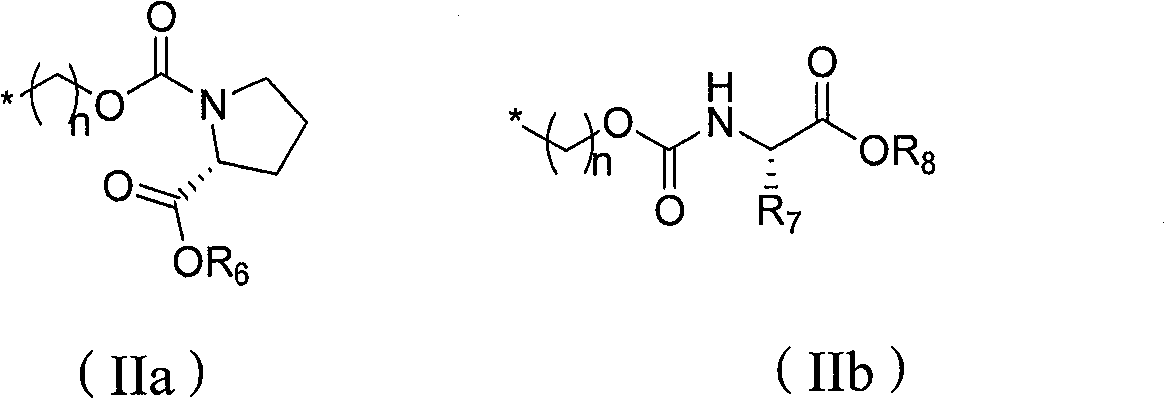
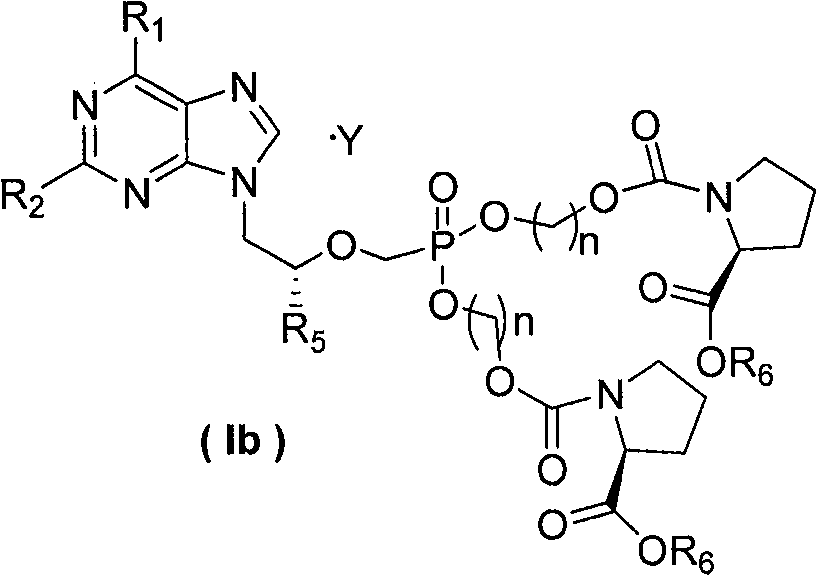
Non-cyclic nucleoside phosphonate compound and its composition, prepn process and use
A technology of acyclic nucleoside phosphonate and cyclic nucleoside phosphonate, which is applied in the fields of pharmacy, drug synthesis and pharmacology, and can solve problems such as unstable chemical properties, high toxicity, and low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0092] Preparation Example 1N-(Chloromethoxycarbonyl)-L-Proline Methyl Ester Preparation
[0093]
[0094] At -5~0℃, N 2 Under protection, a mixture of proline methyl ester hydrochloride (5.80g, 0.035mol), DIEA (11.3g, 0.088mol), DMAP (0.086g, 0.70mmol) and dichloromethane (20mL) was added dropwise to chlorine Chloromethyl formate (4.70g, 0.036mol) and dichloromethane (50mL) mixed solution, dropwise finished, slowly rise to room temperature, continue to react for 2 hours, add ethyl acetate (60mL) to dilute, successively wash with saturated sodium bicarbonate Aqueous solution (90mL×3), 1 equivalent of hydrochloric acid (90mL×3), washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, and the residue was subjected to column chromatography (petroleum ether:ethyl acetate=4:1 ) afforded the title product (6.64 g, 85.6%). 1 H NMR (CDCl 3 ): 5.65-5.84(m, 2H), 4.43(m, 1H), 3.75(s, 3H), 3.60(m, 2H), 2.24(m, 1H), 2.03(m, ...
preparation example 2-8
[0097]
[0098] Preparation example
preparation example 9
[0099] Preparation Example 9 Preparation of 2-bromoethyl chloroformate
[0100]
[0101] After triethylamine (16.2g, 0.16mol) was mixed with dichloromethane (70mL), it was added dropwise to 2-bromoethanol (20.0g, 0.160mol), solid phosgene (15.8g, 0.053mol) and In the dried dichloromethane (320mL) mixture, react at 0°C for 2 hours, then rise to room temperature and react for 2 hours, filter, extract with dichloromethane (150mL), wash with water (80mL×2), and saturated sodium chloride solution After washing, it was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain the title product (17.1 g, 57.1%). 1 H NMR (CDCl 3 ): 4.60 (t, J = 6.31 Hz, 2H), 3.56 (t, J = 6.31 Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com