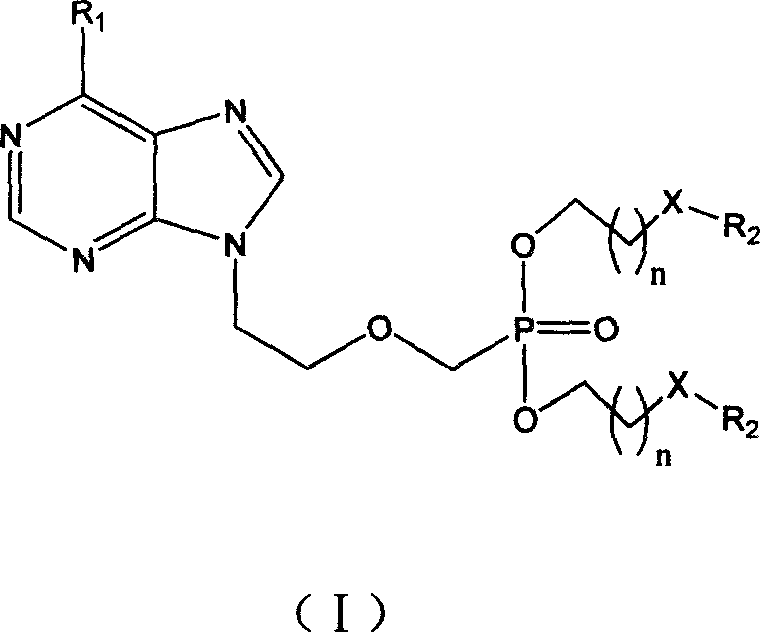
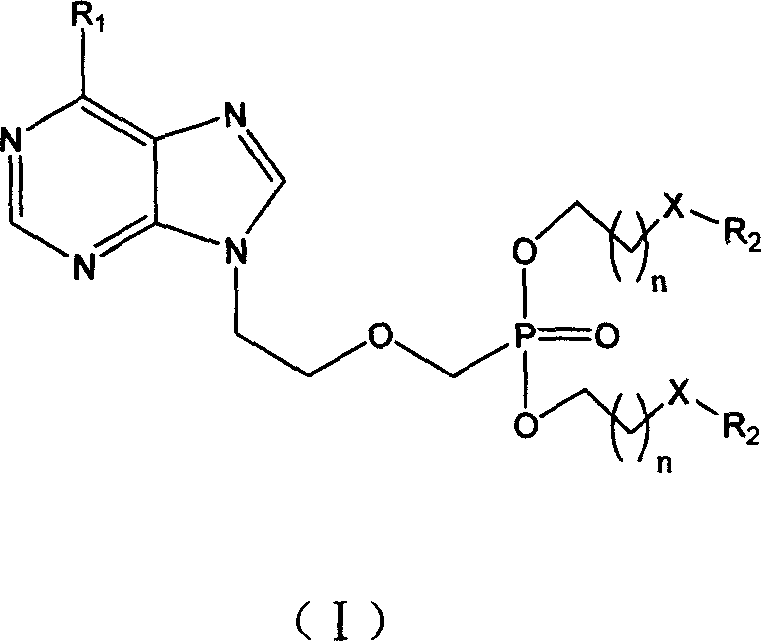
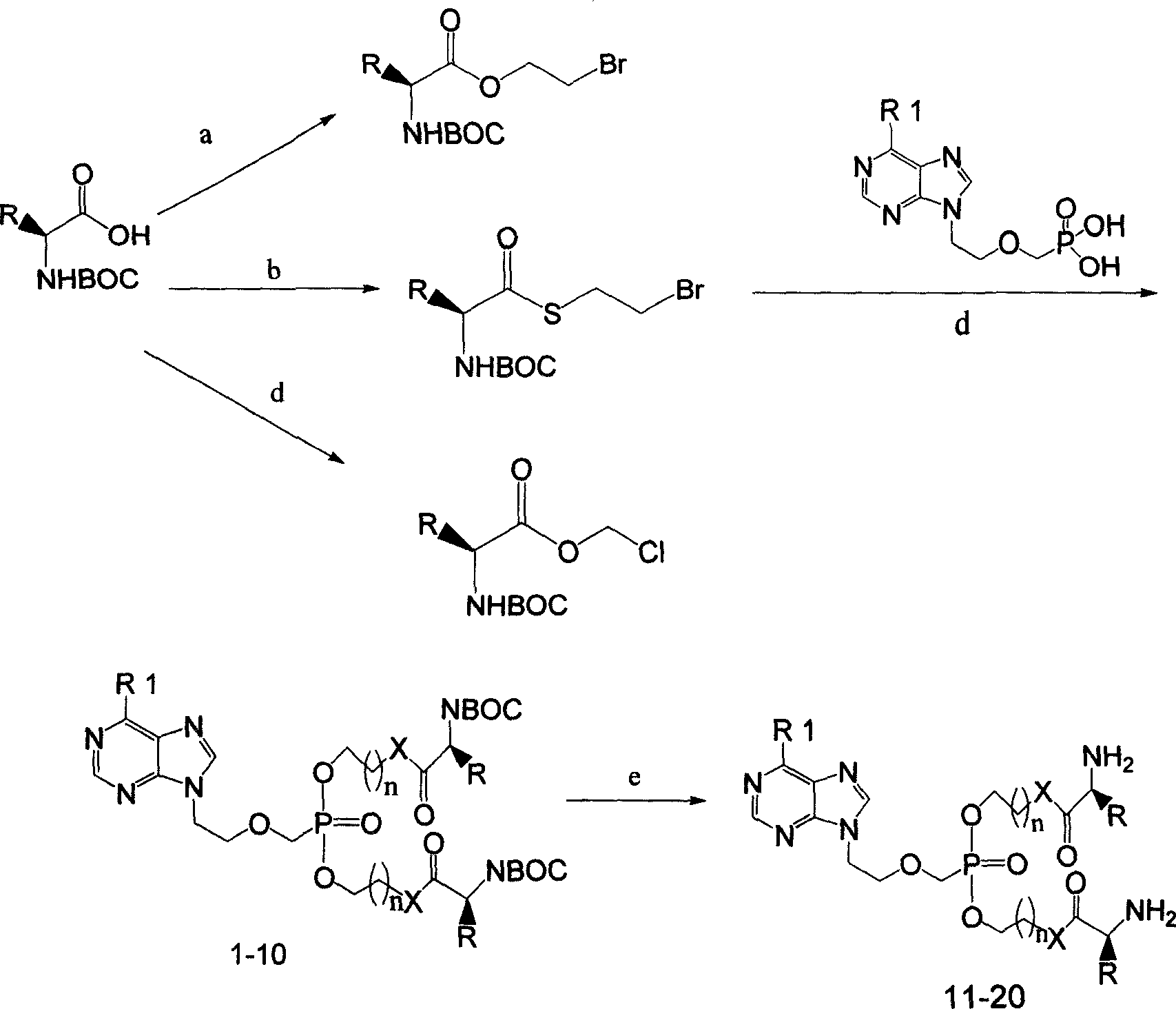
Preparation method and use for purine compounds double amino acid ester
A double amino acid ester and compound technology, applied in the field of medicinal chemistry and antiviral infection therapeutics, can solve the problems of highly sensitive hydrolysis reaction, inability to effectively increase drug concentration at the site of action, unstable chemical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: (2S, 2'S)-9-{2-[O, O'-bis[(2-tert-butoxycarbonylamino-3-methylpentanoyloxy)ethyl]phosphonomethoxy base] ethyl} adenine (compound 1)
[0094] 1.1 Preparation of (1S)-tert-butyl-1-[(2-bromoethoxy)carbonyl]-2-methylbutylcarbamate (a)
[0095]
[0096] Dissolve N-tert-butoxycarbonyl-L-isoleucine (5.00g, 0.02mol), 2-bromoethanol (2.98g, 0.024mol) in 200ml of dry dichloromethane, cool to 10°C in an ice-water bath , added N,N-dimethylaminopyridine (2.92 g, 0.023 mol) in portions. After the addition, the system was kept warm and stirred for 15 minutes. Then a solution of dicyclohexylcarbodiimide (4.53 g, 0.022 mol) in 30 ml of dichloromethane was slowly added dropwise. After the addition, the reaction temperature was naturally raised to room temperature for 12 hours. The insoluble matter in the system was filtered, and the solvent was distilled off. 100 ml of ethyl acetate was added to the obtained residue, and the solids in the system were fully settled at l...
Embodiment 2
[0102] Example 2: (2S, 2'S)-9-{2-[O, O'-bis[(2-tert-butoxycarbonylamino-acetoxy)ethyl]phosphonomethoxy]ethyl} Preparation of adenine (compound 2)
[0103] 2.1: Preparation of tert-butyl-1-[(2-bromoethoxy)carbonyl]methylcarbamate (a)
[0104]
[0105] Dissolve N-tert-butoxycarbonyl-glycine (3.70g, 0.021mol), 2-bromoethanol (3.47g, 0.028mol) in 200ml of dry dichloromethane, cool to 10°C in an ice-water bath, and add N , N-Dimethylaminopyridine (3.10 g, 0.025 mol). After the addition, the system was kept warm and stirred for 15 minutes. Then a solution of dicyclohexylcarbodiimide (4.53 g, 0.022 mol) in 30 ml of dichloromethane was slowly added dropwise. The subsequent process was similar to the synthesis of compound 1.1. 3.21 g of a colorless oil (a) was obtained, with a yield of 54.39%.
[0106] 2.2: (2S,2′S)-9-{2-[O,O′-bis[(2-tert-butoxycarbonylamino-acetoxy)ethyl]phosphonomethoxy]ethyl}adenine (Compound 2) Preparation
[0107]
[0108] At room temperature, (a) (0.9...
Embodiment 3
[0110] Example 3: (2S, 2'S)-9-{2-[O, O'-bis[(2-tert-butoxycarbonylamino-3-methylbutyrylthio)ethyl]phosphonomethoxy Preparation of ]ethyl}adenine (compound 3)
[0111] 3.1: Preparation of (S)-2-tert-butoxycarbonylamino-3-methylmonothiobutanoic acid (a)
[0112]
[0113] Dissolve N-tert-butoxycarbonyl-L-valine (2.50g, 0.0115mol) in 25ml of dry tetrahydrofuran, cool to -15°C in an ice-salt bath, add N-methylmorpholine (11.54g, 0.0575 mol) and isobutyl chloroformate (1.74g, 0.0126mol), the system was kept stirring for 30 minutes. Keep the internal temperature of the reaction system below -15°C, feed self-made hydrogen sulfide gas for 1.5-2 hours, until the hydrogen sulfide gas in the system is absorbed to saturation, then keep the temperature for another 2 hours to complete the reaction. Add 40ml of anhydrous ether, adjust the pH of the system to 3 with 0.1M hydrochloric acid, separate the organic layer, wash with 2 x 20ml of water, 2 x 20ml of saturated brine, dry over anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com