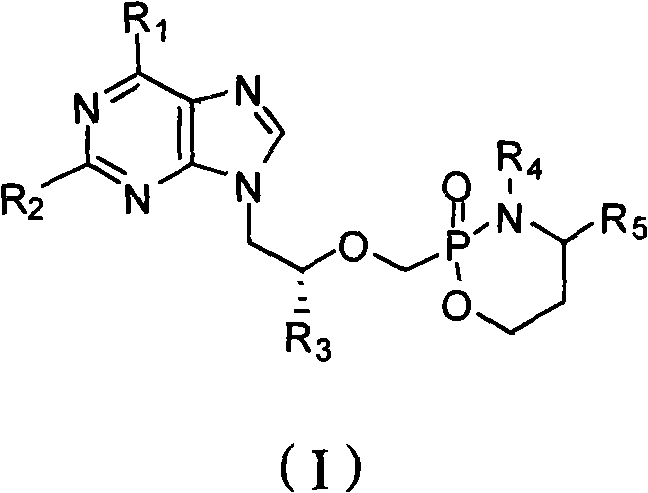
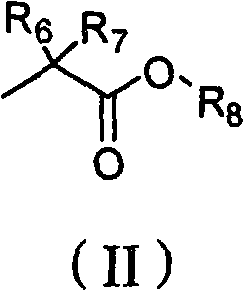
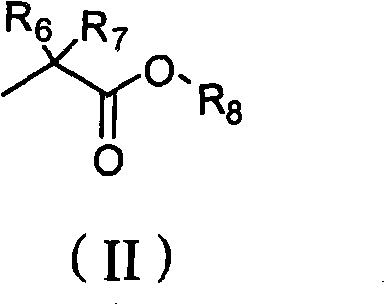
New acyclic nucleoside phosphonate compound as well as composition, preparation method and application thereof
A non-cyclic nucleoside phosphonate and cyclic nucleoside phosphonate technology, which is applied in the fields of pharmacy, drug synthesis and pharmacology, can solve the problem of unstable chemical properties, inability to effectively increase drug concentration at the site of action, and highly sensitive hydrolysis reactions And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] The preparation of embodiment 1 L-valine methyl ester hydrochloride
[0096]
[0097] At -10°C, valine (10g, 85.5mmol) was dissolved in methanol (120ml), and thionyl chloride (22.4ml, 307mmol) was slowly added dropwise. Hour. After cooling down to room temperature, the methanol was distilled off under reduced pressure, and the residue was recrystallized from a methanol-ether mixture to obtain the title product (10 g, 70%). 1 H NMR (CDCl 3 ): 8.8(br, 3H), 4.0(t, 1H), 3.8(s, 3H), 2.5(m, 1H), 1.1(d, 6H).
[0098] The compounds listed in the following table can be prepared from various natural amino acids and unnatural amino acids according to the same steps as in Example 1.
Embodiment 2-10
[0100]
[0101] Reality
[0102] H)
Embodiment 11
[0103] The preparation of embodiment 11 N-benzyl-L-valine methyl ester
[0104]
[0105] At 0°C, L-valine methyl ester hydrochloride (7g, 41.8mmol) was dissolved in methanol (150ml), then sodium cyanoborohydride (2.63g, 41.8mmol) was added, and then benzene was added dropwise Formaldehyde (4.43g, 41.8mmol) was raised to room temperature and reacted overnight. Cool to 0°C, acidify the reaction solution with concentrated hydrochloric acid until pH = 1, stir at the same temperature for 3.5 h, distill under reduced pressure, add water, adjust pH = 9 with saturated sodium carbonate solution, extract twice with ethyl acetate, wash with water, After washing with saturated sodium chloride, it was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness under reduced pressure. The residue was subjected to column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain the title product (6 g, 64.9%). 1 H NMR (CDCl 3 ): 7.2-7.4(m, 5H), 3.82, 3.6(dd, 2H), 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com