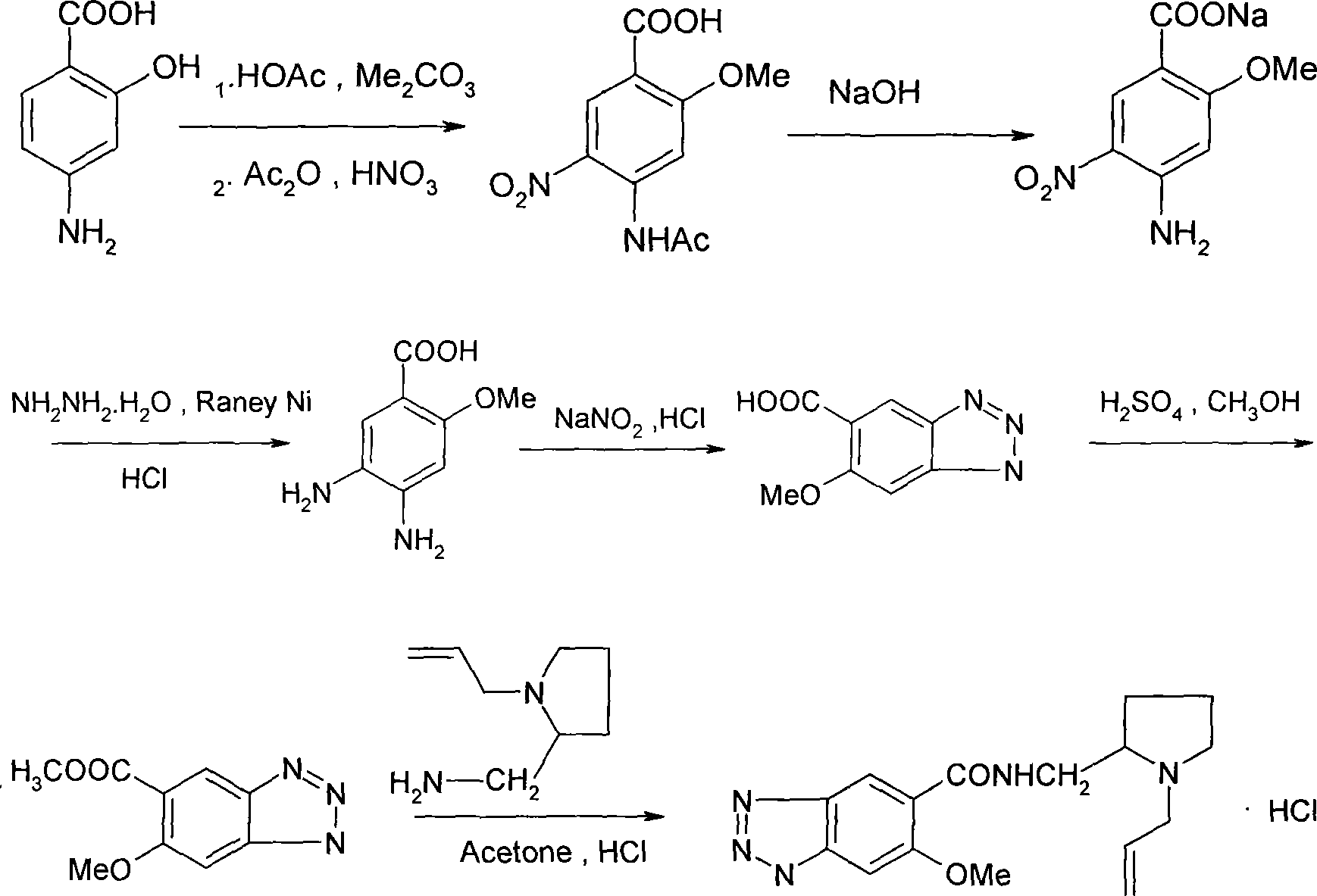
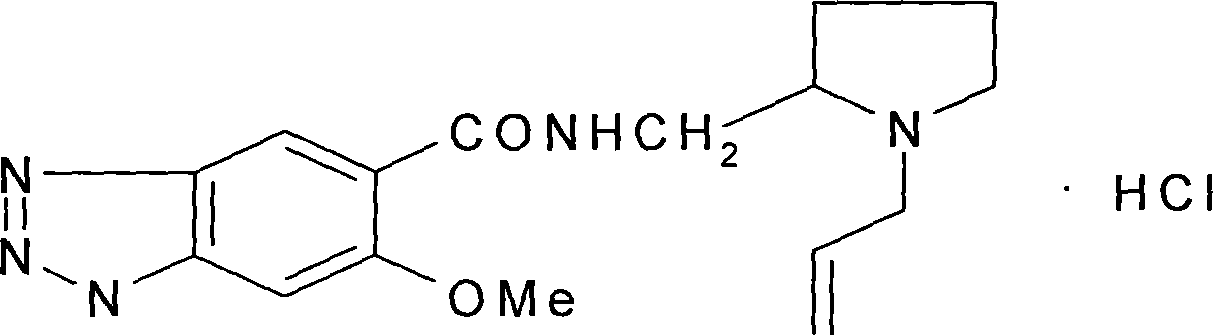Prepn process of alizapride hydrochloride
A technology of hydrochloride and sodium nitrobenzoate is applied in the field of preparation of alipride hydrochloride to achieve the effect of reducing the amount of three wastes treated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Preparation of intermediate 2-methoxy-4-amino-5-nitrobenzoic acid:
[0031] In the reaction flask, add 31.5 grams of 4-aminosalicylic acid and 80 ml of glacial acetic acid, add 20 grams of dimethyl carbonate under stirring, heat up and reflux for 4-10 hours, then add 60 grams of acid anhydride after cooling, heat and reflux for 4-9 hours, After cooling, add 20ml of nitric acid dropwise, stir at room temperature overnight after dropping, pour into crushed ice and stir to precipitate a solid, filter and wash with water to obtain an intermediate, which does not need to be dried for later use.
[0032] 2. Preparation of intermediate 2-methoxy-4-amino-5-nitrobenzoate sodium:
[0033] In the reaction bottle, add the intermediate obtained in the previous step, and then add aqueous sodium hydroxide solution to raise the temperature and reflux for 2-5 hours, then cool and filter to obtain the intermediate sodium 2-methoxy-4-amino-5-nitrobenzoate, which is directly used in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com