Electrolytic synthesis method for 1,5-diamino-4,8-dihydroxyanthraquinone by one-step method
A technology of dihydroxyanthraquinone and electrolytic synthesis, which is applied in electrolytic process, electrolytic components, electrolytic organic production, etc., can solve the problems of high production cost, environmental pollution, large equipment investment, etc., and achieves low production cost, short process route, The effect of less pollution from the three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
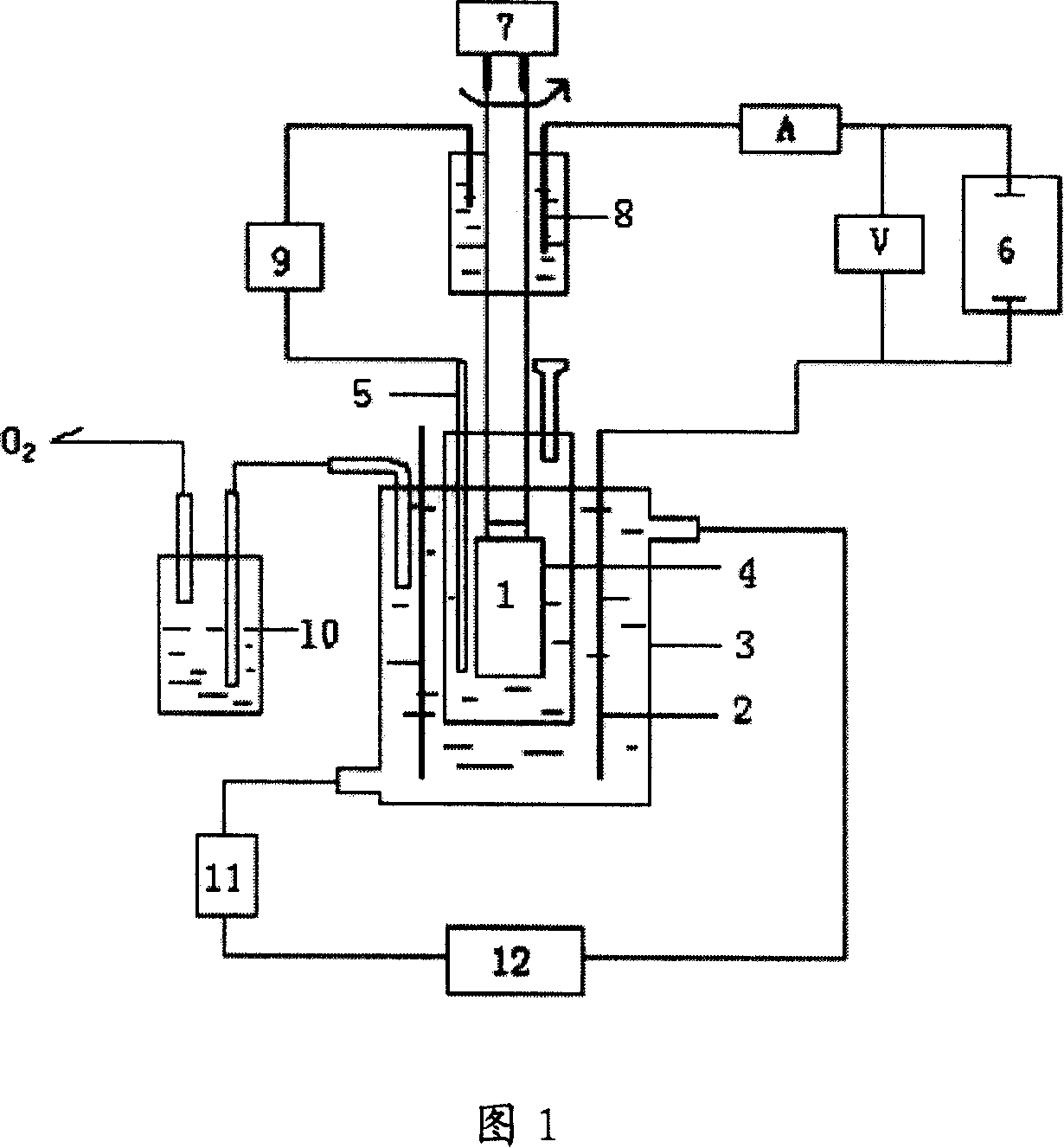
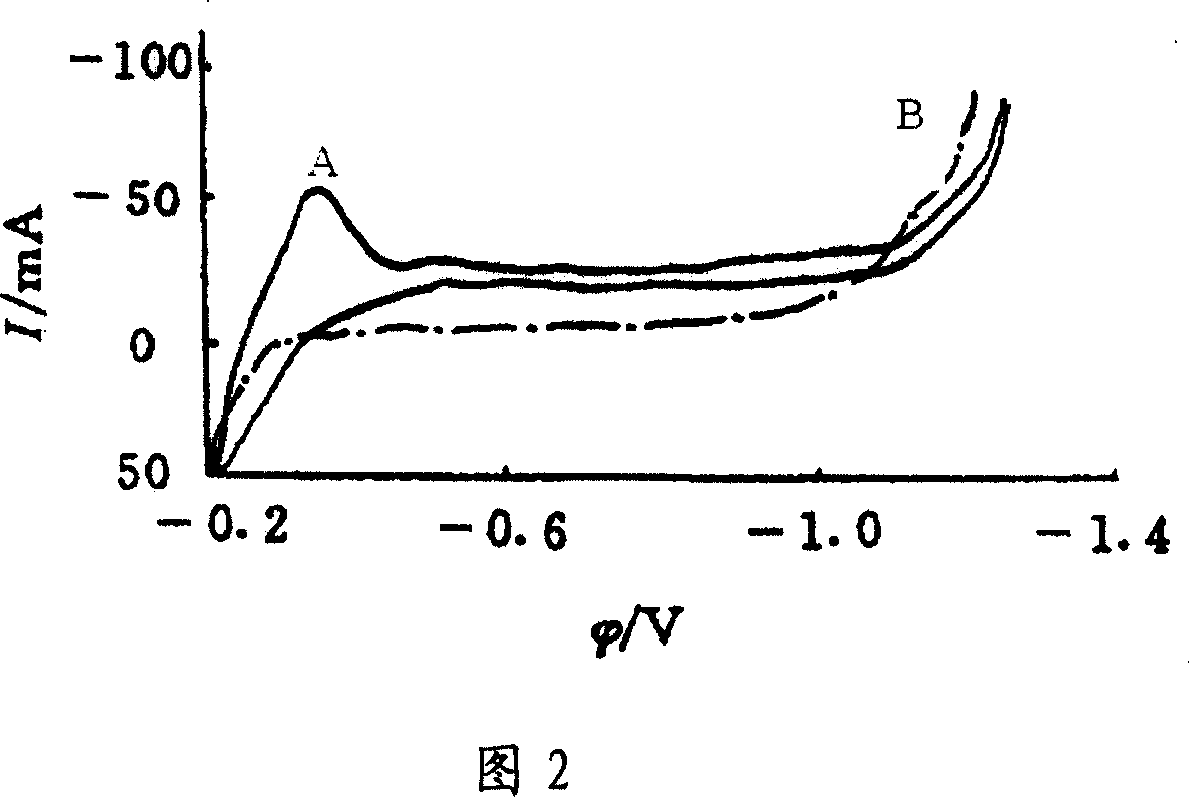
[0030] At first do blank test, adopt the device shown in accompanying drawing 1, amalgam copper electrode (Cu / Hg) is cathode, platinum plate is anode, the same solution dynamic hydrogen electrode is reference electrode, negative, anode liquid is 12.75mol / L sulfuric acid solution at a temperature of 140°C, using a Potentistat / galvanostat Model 273A from Princeton, USA, at a scan rate of 100mv / s for cyclic voltammetry scanning. The result is curve B in FIG. 2 .
[0031] The amalgamated copper electrode is used as the cathode, the platinum sheet is used as the anode, the same solution dynamic hydrogen electrode is used as the reference electrode, and 12.75mol / L sulfuric acid+0.00671mol / L 1,5-dinitroanthraquinone is used as the catholyte (ie, catholyte The final concentration of sulfuric acid in the medium is 12.75mol / L, and the final concentration of 1,5-dinitroanthraquinone is 0.00671mol / L, and other identical expressions have the same meaning), 12.75mol / L sulfuric acid solutio...
Embodiment 2~5
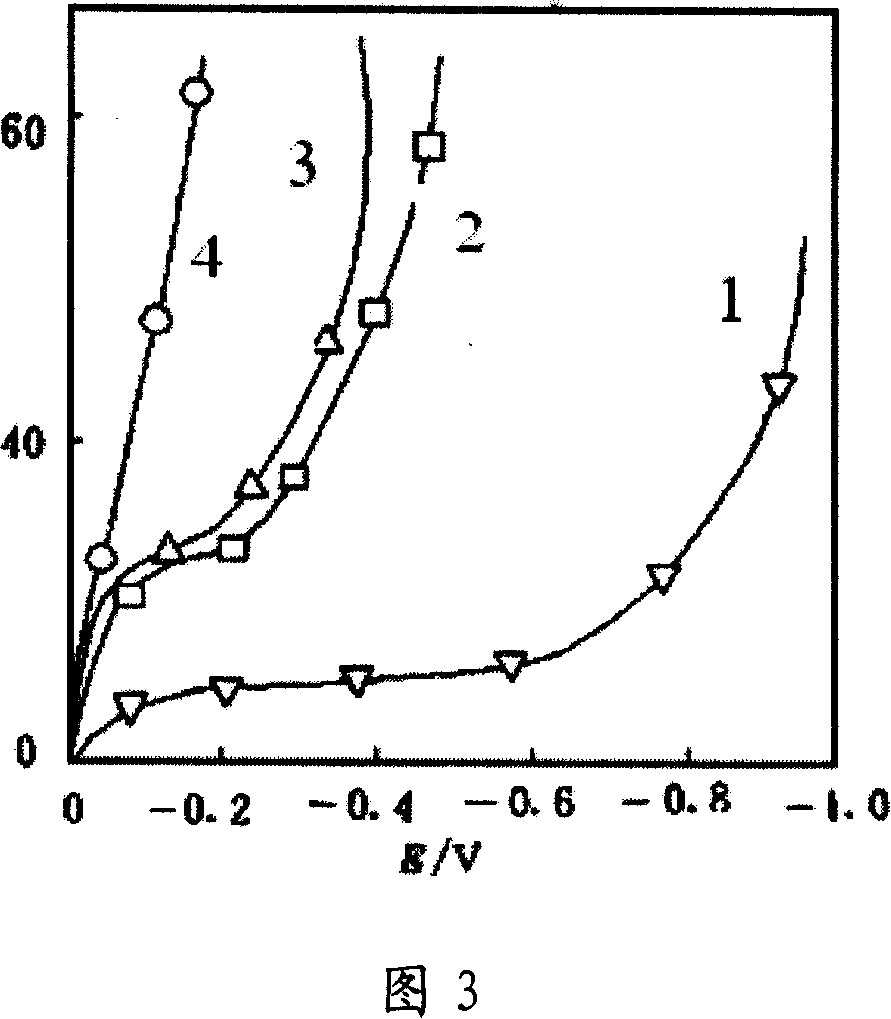
[0035]Embodiments 2-5 use Cu / Hg, Cu, Zn-Ni-Cu and Zn electrodes as cathodes respectively, platinum sheets as anodes, the same solution dynamic hydrogen electrode as reference electrode, catholyte is 12.75mol / L sulfuric acid+0.00671mol / L 1,5-dinitroanthraquinone, the anolyte is 12.75mol / L sulfuric acid solution, and the temperature is 140°C. Use the Potentistat / galvanostatModel 273A of Princeton, USA to perform steady-state polarization scanning. The results are shown in Figure 3 Curve 1, Curve 2, Curve 3, Curve 4 in.
Embodiment 6~9
[0037] With Cu / Hg as cathode, platinum sheet is as anode, and the same solution dynamic hydrogen electrode is reference electrode, and in the catholyte of embodiment 6~9, the final concentration of 1,5-dinitroanthraquinone is 0.00671mol / L, The final concentrations of sulfuric acid are 10.20mol / L, 11.90mol / L, 13.60mol / L, and 14.45mol / L, respectively, and the anolyte corresponds to sulfuric acid solutions of the same concentration, and the temperature is 140°C. / galvanostatModel 273A, respectively for steady-state polarization curves, the results are curve 1, curve 2, curve 3, curve 4 in Figure 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com