Vaccine conjugate including a human chorionic gonadotropin beta subunit antigen linked to an anti-mannose receptor (mr) antibody
A chorionic gonadotropin, monoclonal antibody technology, used in antibody mimics/scaffolds, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, for targeting specific cell fusion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Example 1: Production of βhCG-B11
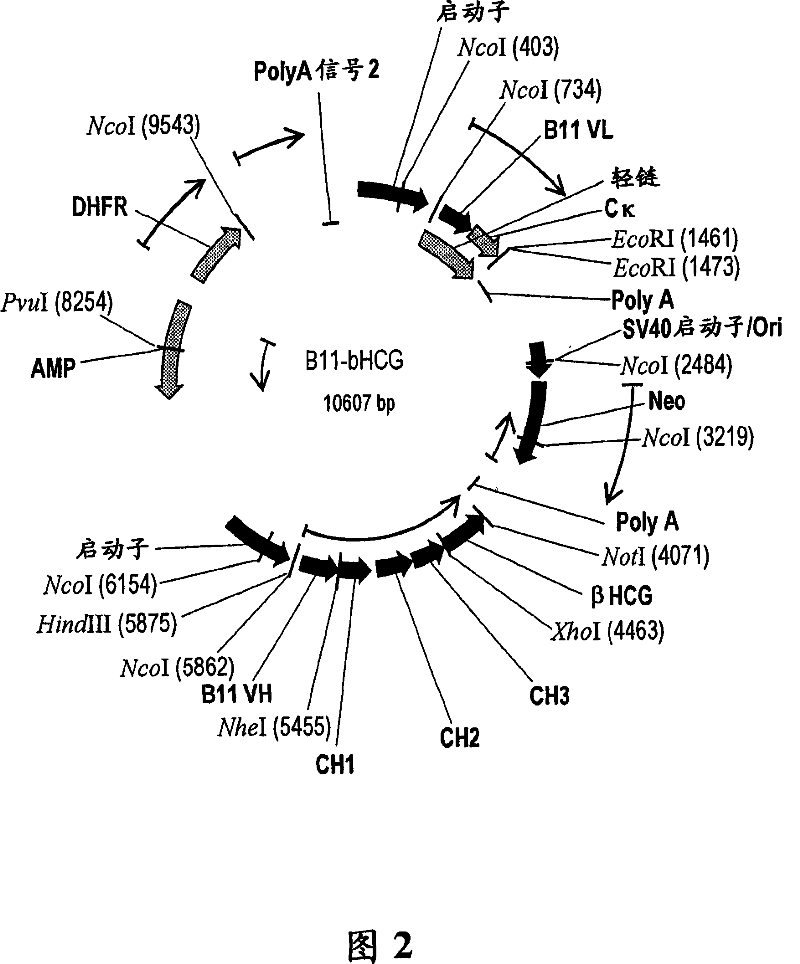
[0245] Vaccine conjugate design: The construct is produced by linking the βhCG antigen with B11, a complete human antibody that binds to the human macrophage mannose receptor on dendritic cells. Through the genetic fusion method, the heavy chain of the antigen and the antibody are covalently bonded to complete the bonding, as shown in Figure 3.
[0246] Recombinant expression of βhCG-B11 vaccine conjugate: As shown in Figure 2, a plasmid containing the neomycin gene and the dihydrofolate reductase gene is generated, which contains the antibody B11 in the heavy chain CH 3 Region-fused βhCG coding sequence (SEQ ID NO: 9 and 10). Using a standardized protocol (Qiagen Inc, Valencia, CA), the resulting plasmid construct was transfected into CHO cells. The transfected cells were selected in a medium containing the antibiotic G418. Let the cells grow in increasing concentrations of methotrexate to further amplify the expression. After expansion,...
Embodiment 2
[0247] Example 2: Production of B11scfv-βhCG
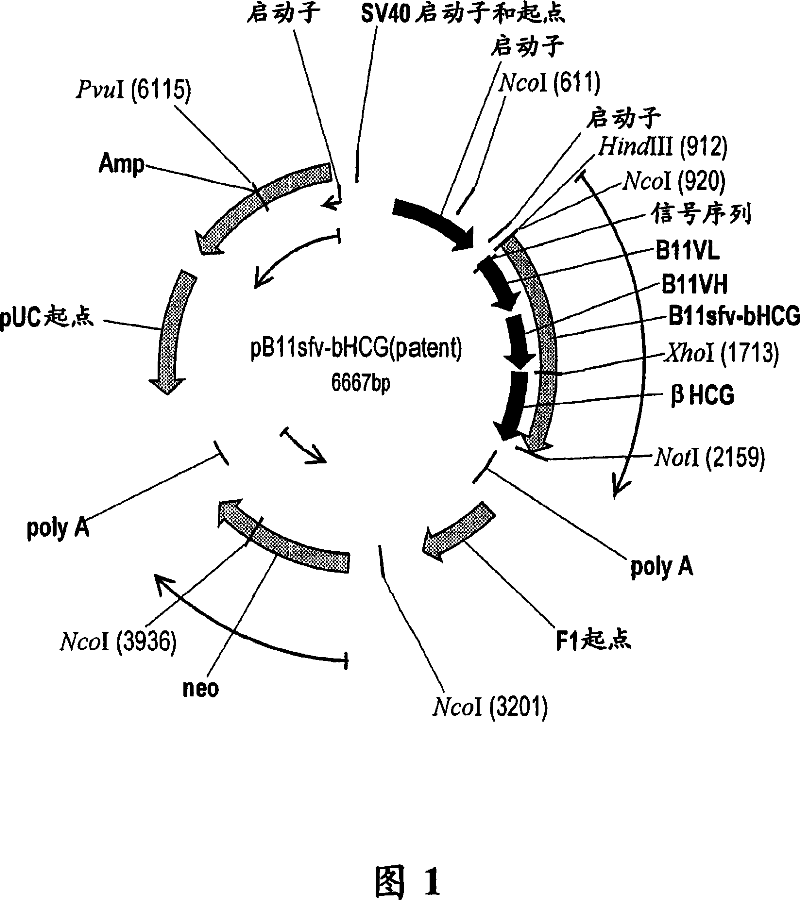
[0248] Vaccine conjugate design: The second construct is produced by linking the βhCG antigen with the B11 single-chain fusion (ScFv). The B11 single-chain fusion (ScFv) is capable of interacting with the mannose receptor of human macrophages on dendritic cells. Body binding and containing intact human B11 antibody V L And V H Fragmented single chain antibody. Through the genetic fusion method, the antigen and the carboxyl end of the B11 ScFv are covalently bonded to complete the bonding, as shown in Figure 1 (referred to as the B11sfv-βhCG construct).
[0249] Recombinant expression of the B11sfv-βhCG vaccine conjugate: As shown in Figure 1, a plasmid containing the B11sfv-βhCG construct (SEQ ID NO: 11 and 12) was generated. Using a standardized protocol (Qiagen Inc, Valencia, CA), the resulting plasmid construct was transfected into mammalian cells. The transfected cells were selected in a medium containing the antibiotic G418. ELIS...
Embodiment 3
[0250] Example 3: Functional characteristics of vaccine conjugates
[0251] The recognition of antibody-targeted vaccines on its associated receptors on the surface of APC is the first step of this drug delivery platform. Flow cytometry studies have confirmed that βhCG-B11 and B11sfv-βhCG constructs specifically bind to MR-expressing cultured human DC (Figure 4).
[0252] The anti-MR antibody was used as a probe to examine the in situ staining of MR on macrophages in human skin DCs and various human tissue sections. Frozen sections of human tissues were stained with anti-MR human antibody B11. The DC present in the epidermal layer of the skin was clearly labeled with the B11 antibody (data not shown). Note that there is a combination with DC in the epidermis of the skin. Furthermore, immunohistochemistry experiments with dendritic cells stained with anti-MR B11 HuMAb in all tissues showed no unexpected cross-reactivity (results not shown). These studies have been repeated with βhC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com