Manganese catalyst of 8-hydroxy quinoline derivative and its uses in olefin epoxidation
A technology of hydroxyquinoline and manganese catalysts, which is applied in the fields of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., and can solve problems such as difficult synthesis, difficult recovery, and low practical value , to achieve high utilization rate, high epoxidation efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
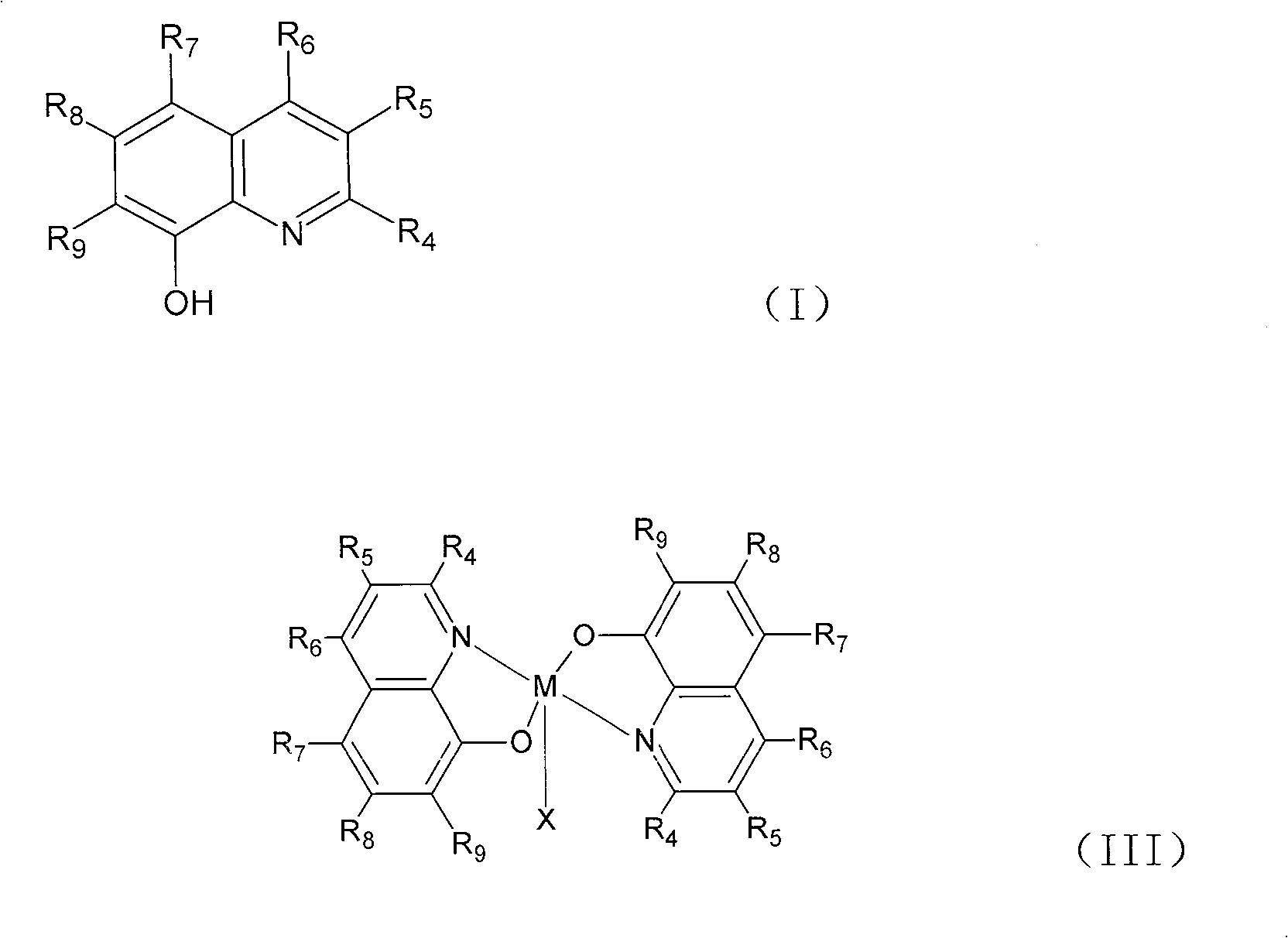
[0027] Embodiment 1: the synthesis of general formula (II) catalyst:
[0028] Dissolve 10mmol of the ligand of general formula (I) in 30-100ml of ethanol or methylene chloride, and add 20ml of Mn(OAc) containing 5mmol Mn(OAc) dropwise under stirring. 2 ethanol solution. Continue stirring at room temperature for 10 to 20 minutes. Filter with suction, wash with ethanol, and dry to obtain a solid that is the catalyst of general formula (II). The product yield is about 90%.
Embodiment 2
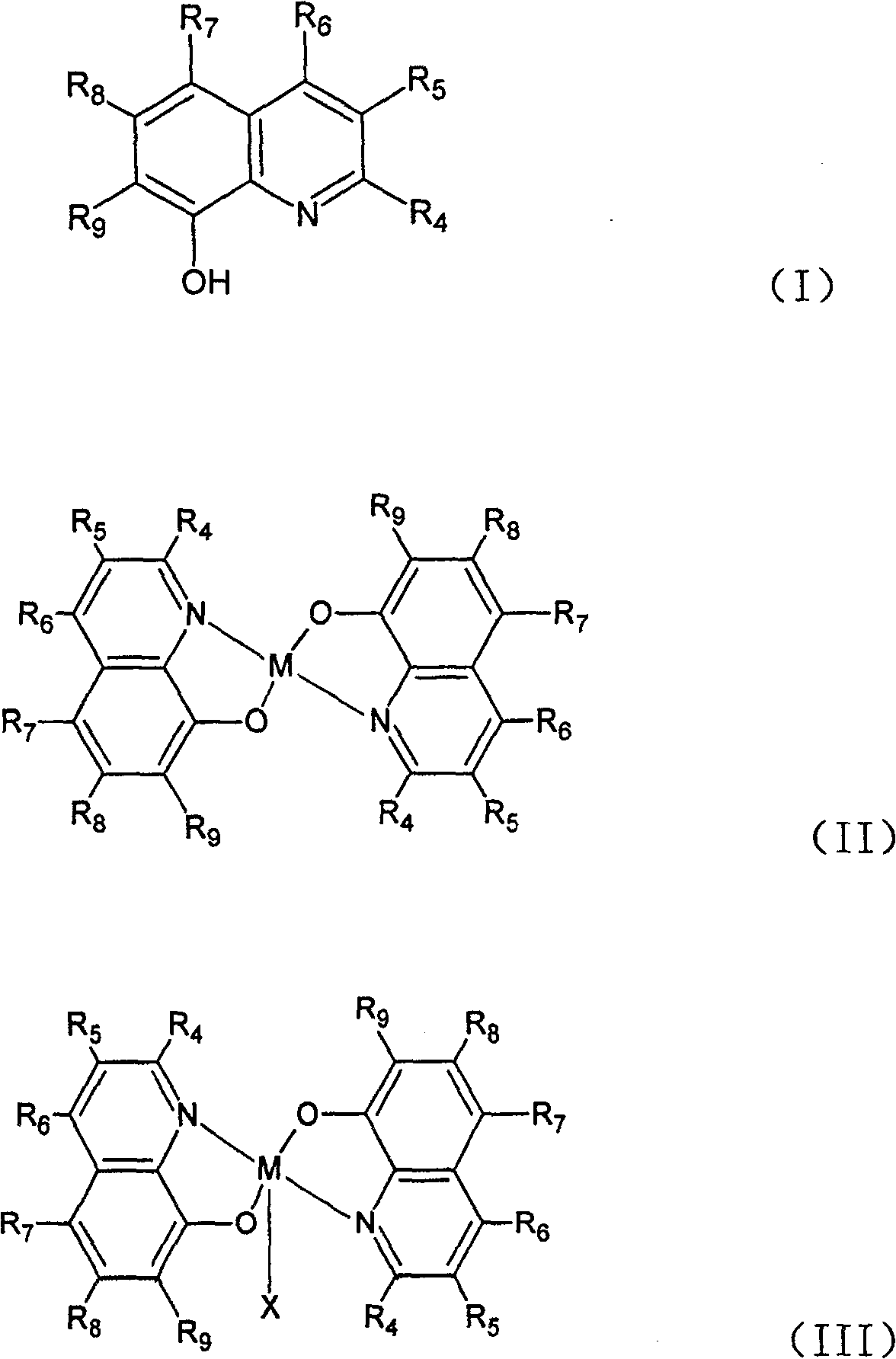
[0029] Embodiment 2: the synthesis of general formula (III) catalyst:
[0030] Dissolve 10mmol of the ligand of general formula (I) in 30-50ml
[0031] In tetrahydrofuran, add 20ml of 5mmol MnCl dropwise under stirring 2 ethanol solution. Then drop in 2-3mmol hydrogen peroxide, then drop in ammonia water to adjust the pH value of the solution to between 6-7 or drop in 4ml containing 10mmolNH 4 Aqueous solution of OAc. Continue to stir or reflux at room temperature for 1 to 2 hours. Filtrate with suction, wash with ethanol, and dry to obtain a catalyst (X=Cl) of general formula (III). The product yield is about 90%.
Embodiment 3
[0032] Embodiment 3: the synthesis of general formula (III) catalyst:
[0033] Dissolve 10mmol of a ligand of general formula (I) in 30-50ml of tetrahydrofuran, and add 20ml of Mn(OAc) containing 5mmol Mn(OAc) dropwise under stirring. 2 ethanol solution. Then add 2-3mmol hydrogen peroxide dropwise; continue to stir or reflux at room temperature for 1 to 2 hours; filter with suction, wash with ethanol, and dry to obtain a solid that is the desired catalyst (X=OAc); the product yield is about 90% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com