Organic electrofluorescent iridium complex material
A technology of phosphorescent materials and iridium complexes, which is applied in the field of iridium complex-based organic electrophosphorescent materials and their synthesis, can solve the problems of reduced lifetime and stability of light-emitting devices, concentration quenching, etc., and achieve reduced phosphorescent lifetime and reduced interaction role, reduce the effect of phase separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
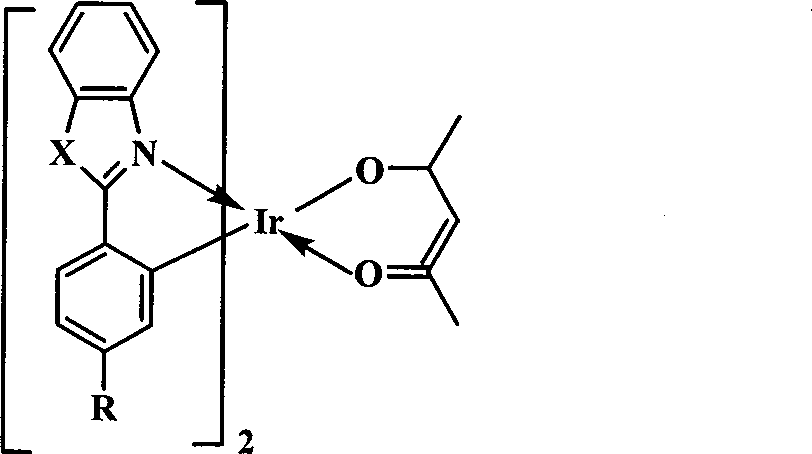
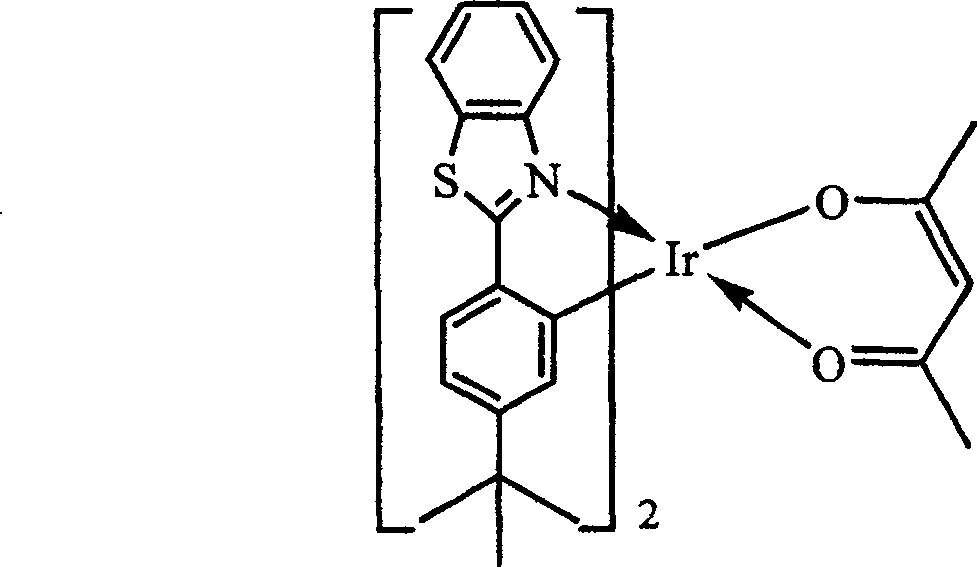
[0024] The organic electrophosphorescent material in this embodiment is an orange-red phosphorescent material, and the purpose of increasing the molecular volume is achieved by introducing a tert-butyl group to the para-position of the 2-phenyl group of the 2-phenylbenzothiazole ligand. Its structural formula is as follows:
[0025]
[0026] In the above structural formula, X is S, R is C(CH 3 ) 3 .
[0027] The preparation process is:
[0028] 1, Preparation of 2-p-tert-butylphenylbenzothiazole (referred to as ligand 1)
[0029] at room temperature, N 2 Under protection, add 0.1mol o-aminothiophenol and 60ml 1-methyl-2-pyrrolidone to a 100ml three-necked flask equipped with a tail gas absorption and condenser tube, then add 0.1mol p-tert-butylbenzene dropwise with a constant pressure funnel Acyl chloride, after the dropwise addition, heated to 100°C for 1 hour to react, the color of the solution changed from yellow to brown, cooled to room temperature, poured the solu...
Embodiment 2
[0034] The organic electrophosphorescent material in this embodiment is an orange-red phosphorescent material, and the purpose of increasing the molecular volume is achieved by introducing an isopropyl group to the para-position of the 2-phenyl group of the 2-phenylbenzothiazole ligand. Its structural formula is as follows:
[0035]
[0036] In the above structural formula, X is S, R is CH(CH 3 ) 2 .
[0037] The preparation process is:
[0038] 1, Preparation of 2-p-isopropylphenylbenzothiazole (referred to as ligand 2)
[0039] Process flow and relevant parameter are identical with embodiment 1, and raw material is substantially identical with embodiment 1, and difference with embodiment 1 is that p-tert-butylbenzoyl chloride in raw material is changed to p-isopropylbenzoyl chloride. The p-cymenoyl chloride used was prepared by refluxing p-cymenic acid with excess thionyl chloride.
[0040] 2. Preparation of complex organic electrophosphorescent material acetylaceton...
Embodiment 3
[0044] The organic electrophosphorescent material in this embodiment is a green phosphorescent material, which is obtained by introducing a tert-butyl group to the para-position of the 2-position phenyl of the 1,2-diphenyl-1H-benzo[d]imidazole ligand. To achieve the purpose of increasing the molecular volume. Its structural formula is as follows:
[0045]
[0046] In the above structural formula, X is ph-N, R is C(CH 3 ) 3 .
[0047] The preparation process is:
[0048] 1. Preparation of 1-phenyl-2-(p-tert-butylphenyl)-1H-benzo[d]imidazole (ligand 3 for short)
[0049] Add 110mmol of o-nitro-N-phenylaniline, 15mmol of zinc chloride, 100ml of benzene and 100mol of p-tert-butylbenzoyl chloride into a 250ml three-necked flask at room temperature, and then heat to reflux for 3 hours. After the reaction is complete, cool to room temperature and filter with suction. The filtrate was evaporated to dryness, and the obtained solid was recrystallized from ethanol to obtain pure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com