Urapidil hydrochloride freeze-dried powder for injection and preparing method thereof
A technology of urapidil hydrochloride and freeze-dried powder, applied in the field of medicine, can solve problems such as drug influence, and achieve the effects of increasing curative effect, eliminating first-pass effect, and being easy to store
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
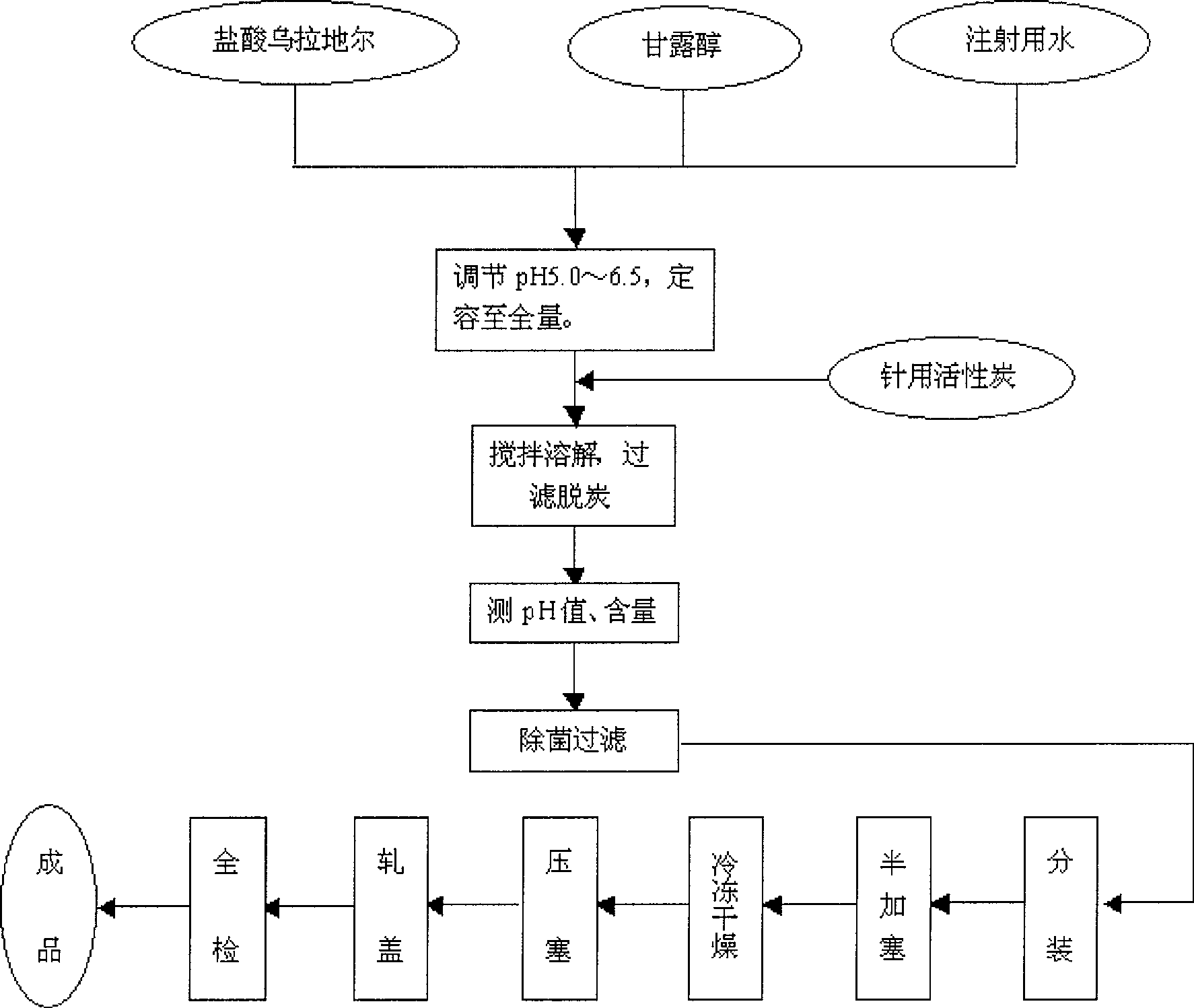
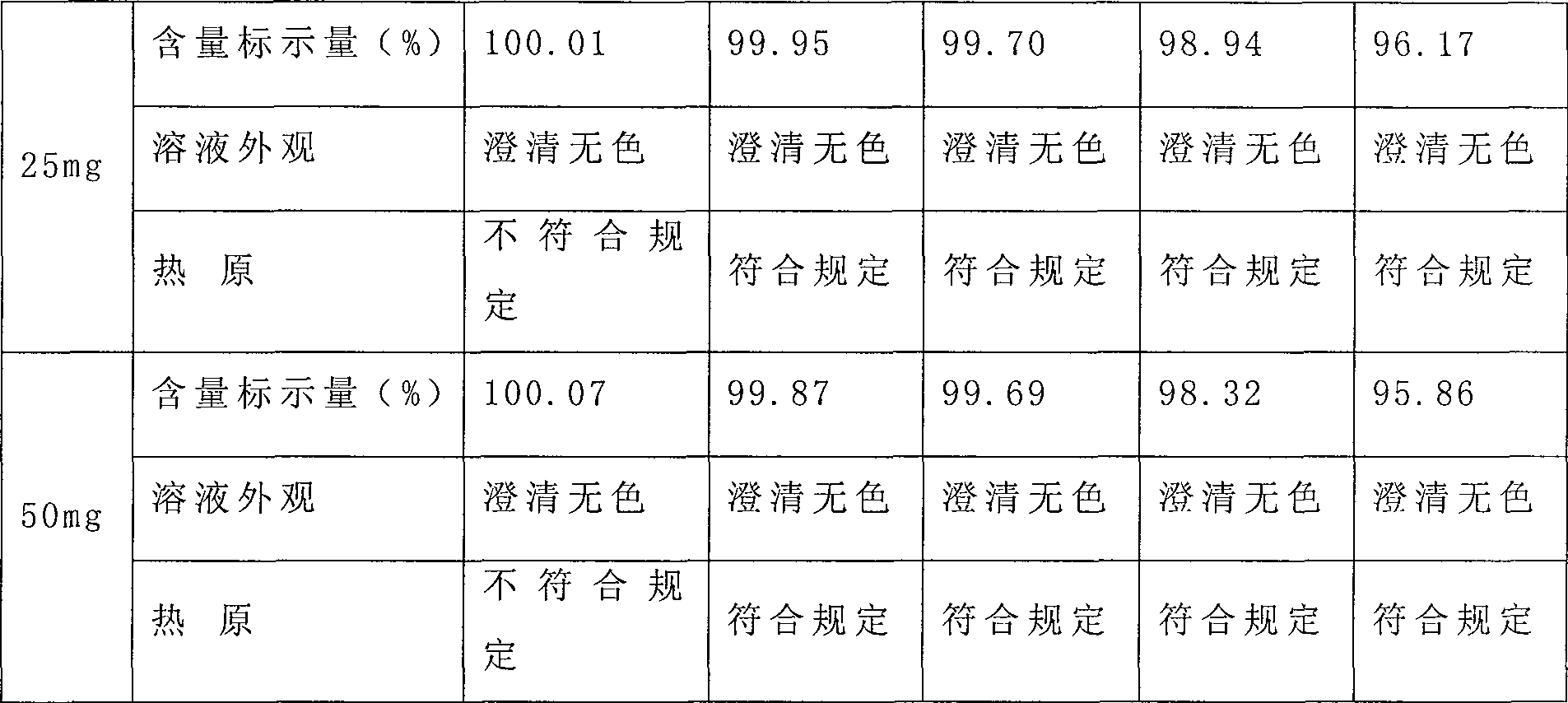
[0052] Add 27.35g of urapidil hydrochloride and 150g of mannitol into the liquid mixing tank, add 1500ml of water for injection and stir to dissolve, adjust the pH value of the solution to 5-6.5 with hydrochloric acid or sodium hydroxide, then add water for injection to 2000ml, add 0.05% (W / V) Medical activated carbon was stirred at room temperature for 40 minutes to decolorize. After decolorization, use a 0.22 μm sterilizing microporous filter membrane to fine filter. The filtrate was measured for pH value and content. The solution was frozen to -55°C for 5 hours, vacuumed to 6Pa after freezing, and slowly heated to -5°C for a heating time of 15 hours, kept at the temperature for 4 hours, then raised to 45°C for 4 hours, and then kept for 2 hours , Plugging, capping, and full inspection are packaged into the finished product warehouse.
Embodiment 2
[0054] Add 54.71g of urapidil hydrochloride and 100g of mannitol into the liquid mixing tank, add 1500ml of water for injection and stir to dissolve, adjust the pH value of the solution to 4 with hydrochloric acid or sodium hydroxide, then add water for injection to 2000ml, add 0.05% (W / V) Medical activated carbon is stirred at room temperature for 15 minutes to decolorize, and after decolorization, use a 0.22 μm sterilizing microporous filter membrane to fine filter, measure the pH value and content of the filtrate, divide it into 50 mg, half-stoppered, and freeze the divided solution to -55°C for 5 hours, vacuumize to 6Pa after freezing, slowly heat to -5°C, heating time is 15 hours, keep the temperature and heat for 4 hours, then raise the temperature to 45°C, heating time is 4 hours, then hold for 2 hours, press Plugging, capping, and full inspection are packaged into the finished product warehouse.
Embodiment 3
[0056] Add 54.71g of urapidil hydrochloride and 100g of mannitol into the liquid mixing tank, add 1500ml of water for injection and stir to dissolve, adjust the pH value of the solution to 6.5 with hydrochloric acid or potassium hydroxide, then add water for injection to 2000ml, add 0.05% (W / V) Medical activated carbon is stirred at room temperature for 15 minutes to decolorize, and after decolorization, use a 0.22 μm sterilizing microporous filter membrane to fine filter, measure the pH value and content of the filtrate, divide it into 50 mg, half-stoppered, and freeze the divided solution To -60°C, 3 hours, vacuumize to 10Pa after freezing, slowly heat to 0°C, heating time 20 hours, keep the temperature and heat for 6 hours, then heat up to 40°C, heating time 6 hours, then keep warm for 3 hours, press the plug , crimping, and full inspection are packaged into the finished product warehouse.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com