Production of high-concentration thymic peptide solution and large-specification thymic peptide preparation
A high-concentration technology of thymosin, which is applied in the field of preparation of thymosin, can solve the problems of easy pollution, waste of pharmaceutical packaging material resources, environmental protection burden, etc., and achieve the effect of simple method, inhibition of microbial reproduction, and maintenance of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
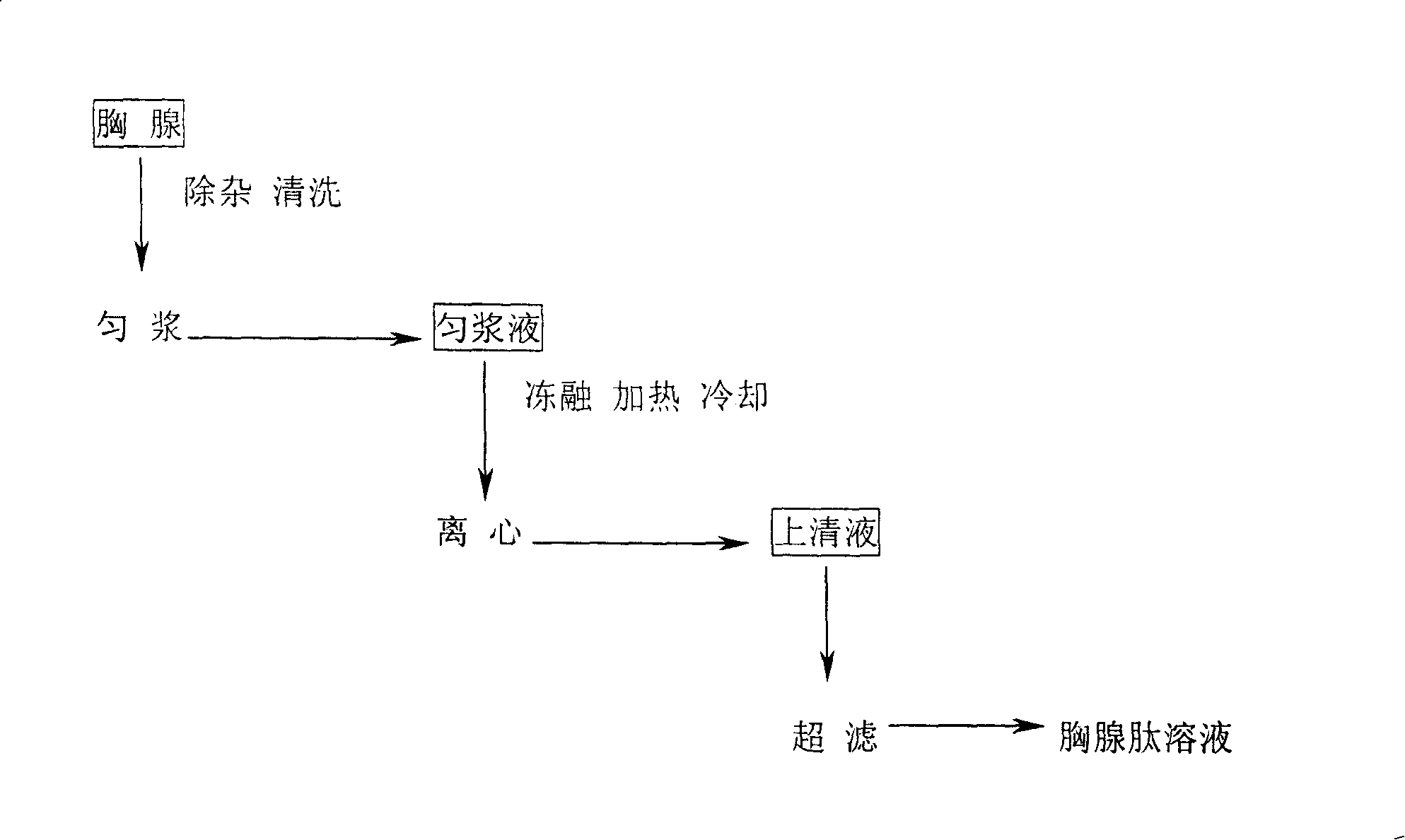
[0028] Get 100Kg of quick-frozen calf thymus and put it in a special water tank to thaw, remove the fat and outer membrane, and get a net weight of 85Kg. The extracted thymus was first washed 3 times with drinking water, and then 3 times with water for injection. After mincing with a meat grinder, add 85L of cold water for injection, and use a colloid mill to homogenize for 2 minutes. The homogenate was frozen at -30°C for 48 hours. After taking it out, it was melted in a water bath at 20°C. It was rapidly heated to 90°C and kept for 3 minutes, and then quickly cooled to room temperature. Centrifuge at 3000rpm for 20 minutes, take the supernatant and filter, and then ultrafilter the filtrate with an ultrafilter with a molecular weight cut-off of 10,000 Daltons, and aseptically collect the permeate, which is the thymosin solution.
[0029] According to the national drug standard WS of thymosin solution 1 -XG-042-2000-2003 detection, the content is 5.9mg / ml, thymosin α 1 Acco...
Embodiment 2
[0031] Get 100Kg of quick-frozen calf thymus and put it in a special water tank to thaw, remove the fat and outer membrane, and get a net weight of 80Kg. The extracted thymus was first washed 3 times with drinking water, and then 3 times with water for injection. After mincing with a meat grinder, add 60L of cold water for injection, and homogenize with a colloid mill for 2 minutes. The homogenate was frozen at -30°C for 24 hours, taken out in a 25°C water bath the next day and thawed, and repeated 3 times. After the third thaw, it was rapidly heated to 80°C and kept for 10 minutes, and then rapidly cooled to room temperature. Centrifuge at 3000rpm for 20 minutes, take the supernatant and filter, and then ultrafilter the filtrate with an ultrafilter with a molecular weight cut-off of 10,000 Daltons, and aseptically collect the permeate, which is the thymosin solution.
[0032] According to the national drug standard WS of thymosin solution 1 -XG-042-2000-2003 detection, the ...
Embodiment 3
[0034] Get 100Kg of quick-frozen calf thymus and put it in a special water tank to thaw, remove the fat and outer membrane, and get a net weight of 83Kg. The extracted thymus was first washed 3 times with drinking water, and then 3 times with water for injection. After mincing with a meat grinder, add 40L of cold water for injection, and homogenize with a colloid mill for 2 minutes. The homogenate was frozen at -30°C for 24 hours, taken out in a 30°C water bath the next day and thawed, and repeated 5 times. After the fifth thaw, it was rapidly heated to 85°C for 5 minutes, and then quickly cooled to room temperature. Centrifuge at 3000rpm for 20 minutes, take the supernatant and filter, and then ultrafilter the filtrate with an ultrafilter with a molecular weight cut-off of 10,000 Daltons, and aseptically collect the permeate, which is the thymosin solution.
[0035] According to the national drug standard WS of thymosin solution 1 -XG-042-2000-2003 detection, the content is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com