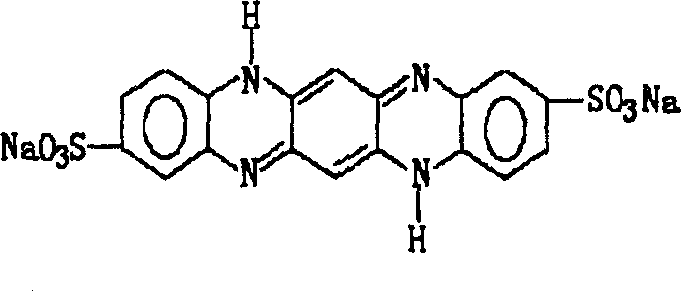
Phacolysin eye medicinal formulation
A pharmaceutical preparation, facolin technology, applied in the field of ophthalmic pharmaceutical preparations, can solve the problems of low product stability, irritation, affecting product quality, etc., and achieve the effects of excellent effect, few side effects and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1. Tablet prescription
[0047] Dosage per 10,000 tablets
Facolin
15g
Bulking agent (taurine)
1000g
200g
100ml
Water for Injection
70ml
[0048] 2. Process
[0049] (1) Pulverization: respectively pulverize Factoline, filler and boric acid through a 60-100 mesh sieve;
[0050] (2) Take ethanol, add water for injection to make it diluted to 40%~60% ethanol liquid, as adhesive;
[0051] (3) Mixing: Add the falcolein, filler and boric acid into the mixing granulator and mix. First dry mix for 8-15 minutes, then add binder and wet mix for 2-5 minutes to make a soft material;
[0052] (4) Granulation: Pass the soft material through a No. 10-14 sieve to granulate;
[0053] (5) Drying: 40~75℃, dry for 3~8 hours;
[0054] (6) Grain sizing: pass through No. 12 to No. 16 sieves after drying;
[0055] (7) Total mixing: 5 to 20 minutes;
[0056] (8) tableting;
[0057] (9)...
Embodiment 2
[0071] Solvent formulation
[0072] Consumption per 10,000 sticks
[0073] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com