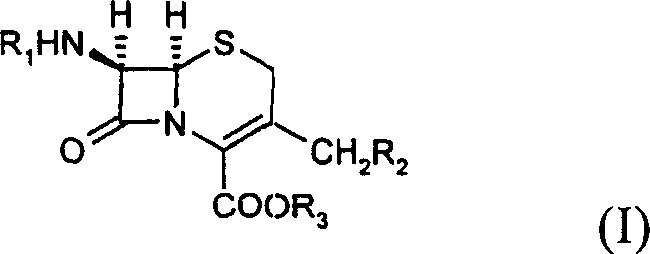
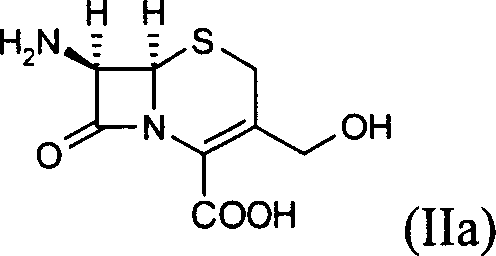
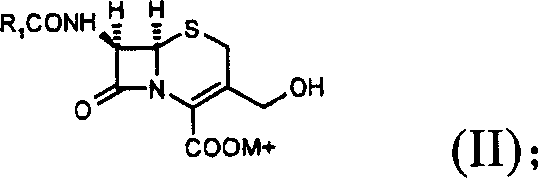
Cephalosporin analog antibiotic preparation method
A cephalosporin and halogen technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, active ingredients of nitro compounds, etc., can solve the problems of environmental pollution of chemical substances, low total product yield, complex reactions, etc., and achieve The effect of reducing reaction steps, high purity of raw materials, and improvement of total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Embodiment 1: the preparation of cefpodoxime axetil (6a)
[0117] This example relates to compound 6a [(6R, 7R)-7-[2-(2-aminothiazol-4-yl)-(Z)-methoxyimino-acetylamino]-3-methoxymethyl-8 -Oxo-5-thio-1-azabicyclo[4.2.0]-oct-2-ene-2-carboxylic acid-1-(isopropylcarbonyloxy) ethyl ester], its preparation process is as follows :
[0118]
[0119] Concrete synthetic steps are as follows:
[0120] (1) (6R, 7R)-7-[2-(2-aminothiazol-4-yl)-(Z)-methoxyimino-acetylamino]-3-hydroxymethyl-8-oxo-5 - Preparation of Sodium Thio-1-azabicyclo[4.2.0]-oct-2-ene-2-carboxylate (Compound 11)
[0121] Suspend 1.15g (5mmol) of compound 8 in 0.3g (2.8mmol) Na at 5-10°C 2 CO 3 Add 1.93g (5.62mmol) of compound 10 to 18ml of acetone aqueous solution, stir for 30 minutes, then naturally warm up to room temperature, react for 5 hours, filter, remove the solid, the filtrate decompresses and recovers acetone to obtain a sticky solid, add acetone, After stirring, a loose solid was obtained, whi...
Embodiment 2
[0129] Embodiment 2: the preparation of cefuroxime axetil (6c)
[0130] This example relates to compound 6c[(6R,7R)-7-[2-(2-furyl)-(Z)-methoxyimino-acetylamino]-3-carbamate-8-oxo - the preparation of 5-thio-1-azabicyclo[4.2.0]-oct-2-ene-2-carboxylic acid-1-acetoxyethyl ester], its preparation process is as follows:
[0131]
[0132]
[0133] Concrete synthetic steps are as follows:
[0134] (1) (6R, 7R)-7-[2-(2-furyl)-(Z)-methoxyimino-acetylamino]-3-hydroxymethyl-8-oxo-5-sulfur-1 - Preparation of sodium azabicyclo[4.2.0]-oct-2-ene-2-carboxylate (compound 15)
[0135] Suspend 3g (13mmol) of compound 8 in 6ml of water at 5-10°C, add 0.7g (6.6mmol) of Na 2 CO 3 , stirred, and added acetone 12ml. In a fume hood, another 15ml of dichloromethane was cooled to 0-5°C, and 3.8g (12.8mmol) of bis-(trichloromethyl)carbonate and 0.02ml of pyridine were added. Add 3.4g (18.2mmol) compound 13, after reacting for 1 hour, slowly raise the temperature to 30°C, continue to stir an...
Embodiment 3
[0143] Embodiment 3: the preparation of cefditoren neopentyl ester (5c)
[0144] This embodiment relates to cefditoren neopentyl ester [(6R, 7R)-{7-[2-(2-aminothiazol-4-yl)-(Z)-methoxyimino-acetylamino]-8- Oxo-5-thio-1-azabicyclo[4.2.0]-oct-2-ene-3-[(cis)-2-(4-methyl-1,3-thiazol-5-yl)ethylene base]-2-carboxylic acid-neivaloyloxymethyl ester (compound 5c)]. Its preparation process is as follows:
[0145]
[0146] The specific steps are as follows:
[0147] (1) (6R, 7R)-7-[2-(2-aminothiazol-4-yl)-(Z)-methoxyimino-acetylamino]-3-hydroxymethyl-8-oxo-5 Preparation of -thio-1-azabicyclo[4.2.0]-oct-2-ene-2-carboxylic acid-neivaloyloxymethyl ester (compound 18)
[0148] According to the example 1 (2) method, compound (11) 2.1g (5.0mmol) was dissolved in 12 milliliters of DMF, reacted with 1.82g neovaloyloxyiodomethyl ester (compound 17) (gas phase assay content: 87.5%), After working up, 2.16 g (yield: 81.97%) of compound 18 were obtained.
[0149] 1 HNMR (DMSO d6, 400MHz)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com