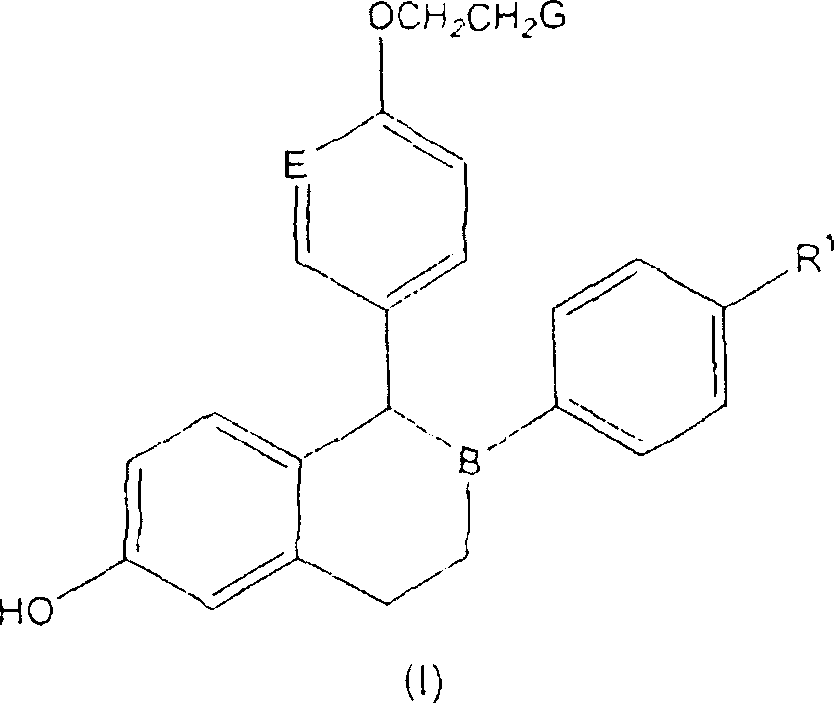
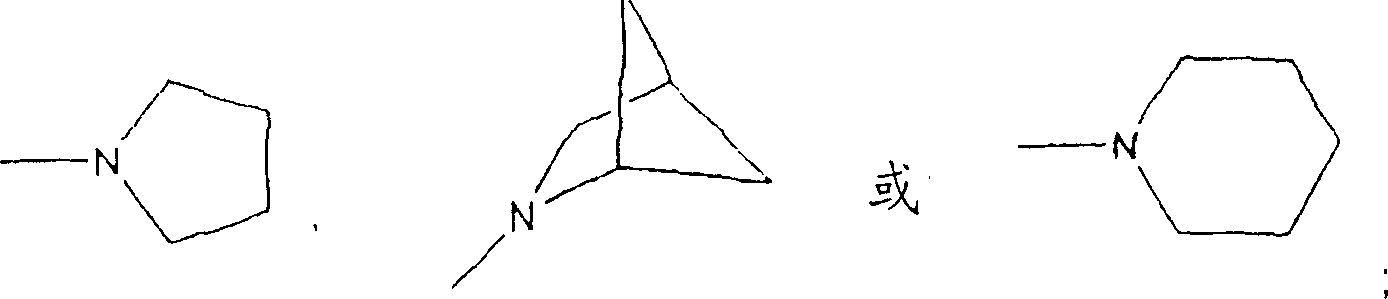
Lasofoxifene tablet and its coating
A technology of lasoxifene tartrate and coating layer, which is applied in directions such as coating, sugar-coated pills, and pill transportation, can solve problems such as unsatisfactory, ineffective manual brushing steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] The following materials used in Example 1 were available from the respective suppliers listed below:
[0097] Avicel TM PH101 FMC Pharmaceutical (Philadelphia, PA)
[0098] (microcrystalline cellulose)
[0099] Lactose Fast Flo TM 316 Foremost Corp. (Baraboo, WI)
[0100] Magnesium stearate Mallinckrodt (St. Louis, MO)
[0101] Hydroxypropyl Cellulose Hercules Inc. (Hopewell, VA)
[0102] Croscarmellose Sodium FMC Pharmaceutical (Philadelphia, PA)
[0103] β-cyclodextrin thiobutyl ether prepared using the method described in US6153746
[0104] Silica Grace Davison (Columbia, MD)
[0105] ProSolv TM 50 Penwest, Patterson, NJ
[0106] (Silicated Microcrystalline Cellulose)
[0107] Conventional wet granulation process of lasofoxifene
[0108] (comparison process)
[0109] Add the following ingredients to the high shear blender in the order listed.
[0110] Lactose 5.000g
[0111] Microcrystalline Cellulose 17.432g
[0112] Croscarmellose Sodium 1.000g
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com