Methods of using macrocyclic modulators of the ghrelin receptor
a macrocyclic modulator and receptor technology, applied in the field of using macrocyclic modulators of the ghrelin receptor, can solve the problems of increasing ghrelin levels, lack of efficacy, and disappointing agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Tethers
A. Standard Procedure for the Synthesis of Tether T9
[0440]
[0441]Step T9-1: To a solution of 2-iodophenol (9-0, 200 g, 0.91 mol, 1.0 eq) in DMF (DriSolv®, 560 mL) is added sodium hydride 60% in mineral oil (3.64 g, 0.091 mol, 0.1 eq) by portions (hydrogen is seen to evolve). The reaction is heated for 1 h at 100° C. under nitrogen, then ethylene carbonate is added and the reaction mixture heated O / N at 100° C. The reaction is monitored by TLC (conditions: 25 / 75 EtOAc / hex; Rf: 0.15. detection: UV, CMA). The reaction mixture is allowed to cool, then the solvent evaporated under reduced pressure. The residual oil is diluted in Et2O (1.5 L), then washed sequentially with 1 N sodium hydroxide (3×) and brine (2×), dried with MgSO4, filtered and the filtrate evaporated under reduced pressure. The crude product is distilled under vacuum (200 μm Hg) at 110-115° C. to provide 9-1.
[0442]Step T9-2: A solution of 9-1 (45.1 g, 0.171 mol, 1.0 eq) and Ddz-propargylamine (9-A, syn...
example 2
Synthesis of Representative Macrocyclic Compounds
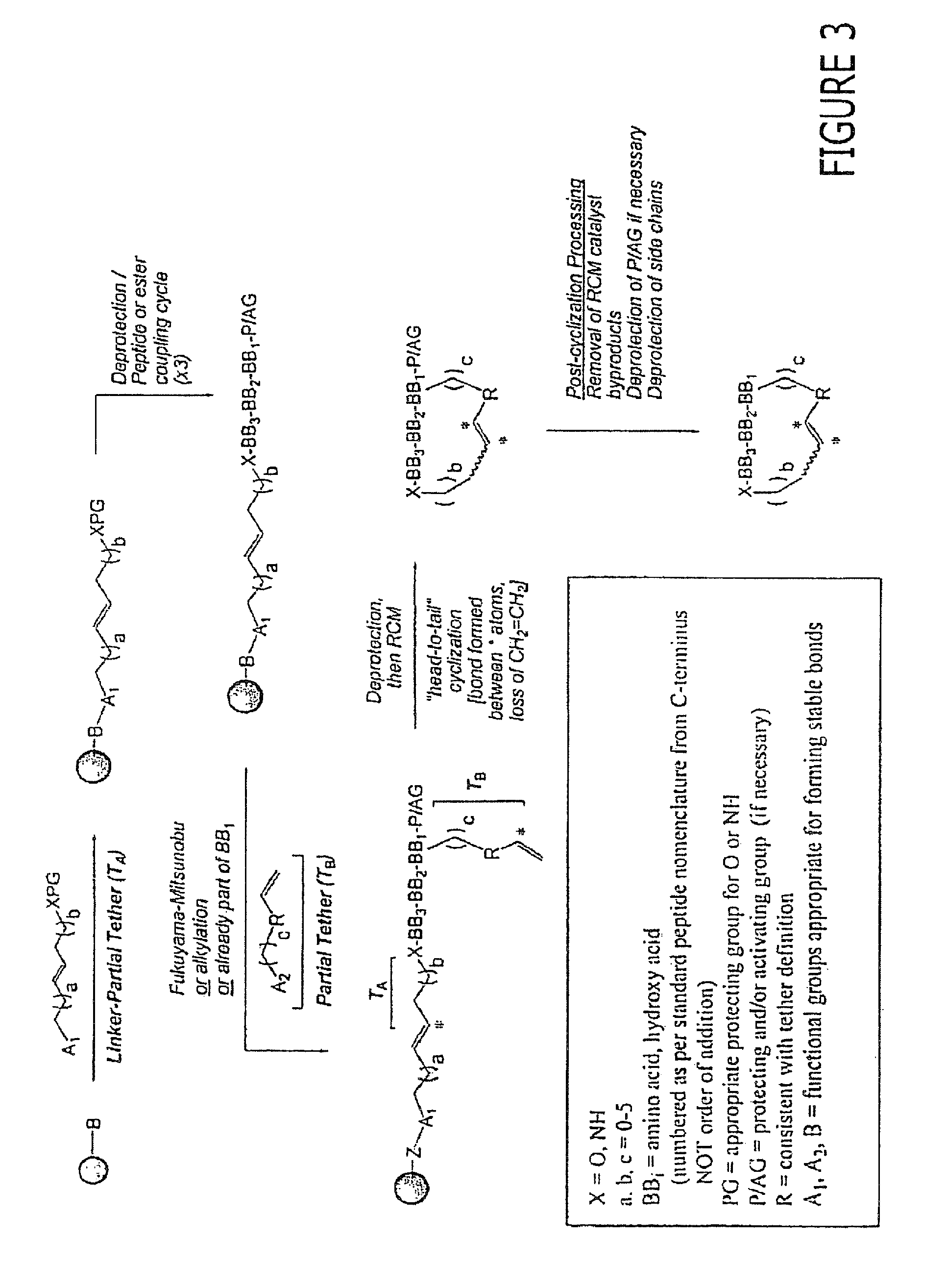
[0556]The following are provided as representative examples for the macrocyclic compounds of the invention. For solid phase methods, all yields are reported starting from 300-325 mg of PS-aminomethyl resin (loading 2.0 mmol / g) unless otherwise noted. Attachment of the first building block, BB3, varies from 100% to 55% for the more difficult residues, typically sterically crowded structures such as Ile or Val. The remaining couplings for BB2 and BB, proceed in an average yield of 80-90%. Attachment of the tether using the Mitsunobu reaction yields from 50-90% of the desired linear precursor. The macrocyclization itself proceeds in an average yield of 20-50%. Minimal loss of yield occurs in post-cyclization processing.
[0557]All the retention time values presented herein are based on the UV portion of the HPLC data. In the HPLC procedure, ELSD and CLND data (not listed) were also procured to further assess purity of the final products, a...
example 3
Alternative Synthetic Strategies
[0592]Alternative synthetic strategies amenable to larger scale synthesis of compounds of the present invention are discussed below.
A. Method LS1 for Representative Large Scale Synthesis of Compounds of the Invention
[0593]
[0594]
[0595]
[0596]Step LS1-A: Synthesis of LS1-8
[0597]
[0598]To alcohol Cbz-T33a (2.4 g, 7.0 mmol, 1.0 eq) in CH2Cl2 (50 mL) were added NBS (1.5 g, 8.4 mmol, 1.2 eq) and PPh3 (2.2 g, 8.4 mmol, 1.2 eq). The mixture was stirred at room temperature O / N and a saturated aqueous NH4Cl solution was added. The aqueous phase was extracted with CH2Cl2 (2×) and the combined organic phases were extracted with a saturated aqueous NH4Cl solution to remove succinimide byproduct. The organic phase was dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography (20% AcOEt, 80% hexanes) to give bromide LS1-8a as a yellow oil (2.6 g, 91%).
[0599]TLC (30% AcOEt, 70% hexanes): Rf=0.56; detection: UV and CMA
[06...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com