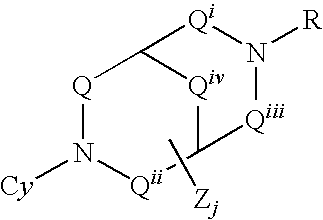
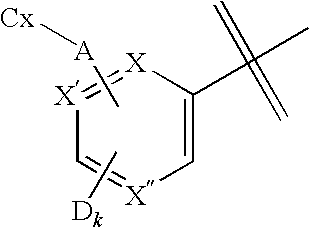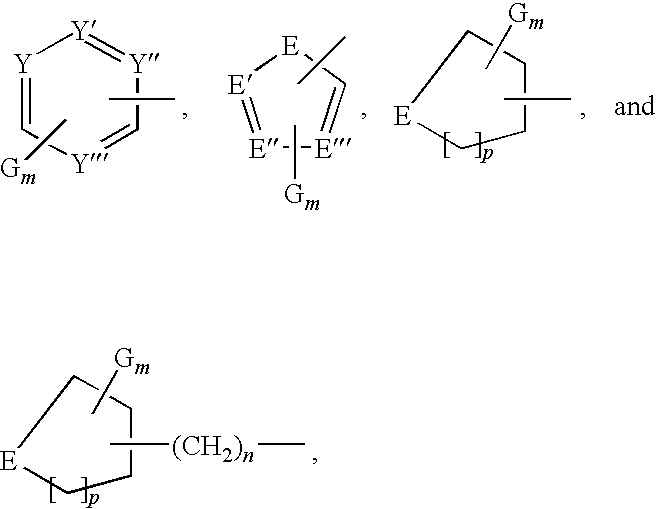Pharmaceutical compositions and methods for use
a technology of nicotinic cholinergic receptor and composition, applied in the field of pharmaceutical compositions, can solve the problems of subtypes with the potential to induce undesirable side effects, no significant associated side effects, etc., and achieve the effects of preventing and suppressing symptoms, no appreciable adverse side effects, and increasing blood pressure and heart ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0109]Sample No. 1 is (1S,4S)-2-(5-(4-methoxyphenoxy)-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane hernigalactarate, which was prepared in accordance with the following techniques:
3-Bromo-5-(4-methoxyphenoxy)pyridine
[0110]To a stirred suspension of sodium hydride (3.0 g of 80% in mineral oil, 100 mmol) in DMF (95 mL) in an ice water bath, 4-methoxyphenol (12.2 g, 96 mmol) was added slowly under a nitrogen atmosphere. The resulting mixture was warmed to ambient temperature and stirred for 1 h. 3,5-Dibromopyridine (15.6 g of 98%, 65 mmol) was added and the mixture was then heated at 85° C. (bath temperature) for 32 h. The mixture was cooled, diluted with water (120 mL), poured into 5N sodium hydroxide (15 mL), and extracted with ether (3×150 mL). The combined ether extracts were dried (Na2SO4), filtered and concentrated by rotary evaporation, to give a light-yellow oil (21.9 g). The oil was diluted with ethanol and rotary evaporated (twice) to remove residual DMF and then diluted with et...
example 2
[0115]Sample No. 2 is (1S,4S)-2-(5-(3-methoxyphenoxy)-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane hernigalactarate, which was prepared in accordance with the following techniques:
3-Bromo-5-(3-methoxyphenoxy)pyridine
[0116]Following a procedure similar to that described for the preparation of 3-bromo-5-(4-methoxyphenoxy)pyridine, 3-bromo-5-(3-methoxyphenoxy)pyridine was prepared using 3-methoxyphenol (2.79 g, 0.023 mole), 75% sodium hydride suspension in oil (0.72 g, 0.023 mol) in dimethyl-formamide (20 mL) and 3,5-dibromopyridine (3.55 g, 0.015 mol). The reaction was worked up using water (50 mL), 5 N NaOH solution (5 mL), and diethyl ether (3×50 mL), and a yellow oil (4.6 g) was obtained as crude product. This oil was purified by column chromatography on silica gel, eluting with cyclohexane-ethyl acetate (92.5:7.5, v / v). Selected fractions containing the product were concentrated via rotary evaporation to give 2.3 g (55%) of a yellow oil.
(1S,4S)-5-(5-(3-Methoxyphenoxy)-3-pyridyl)-2-(t...
example 3
[0121]Sample No. 3 is (1S,4S)-2-(5-(4-fluorophenoxy)-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane dihydrochloride, which was prepared in accordance with the following techniques:
3-Bromo-5-(4-fluorophenoxy)pyridine
[0122]Sodium hydride (1.53 g of 80% in mineral oil, 51 mmol) was slowly added to a solution of 4-fluorophenol (5.6 g, 50 mmol) in DMF (100 mL) as it was stirred and cooled (ice water bath) under a nitrogen atmosphere. The resulting mixture was warmed to ambient temperature and stirred for 1 h. 3,5-Dibromopyridine (5.9 g, 25 mmol) was added and the mixture was then heated at 90° C. (bath temperature) for 62 h. The mixture was cooled, diluted with water (150 mL), poured into 5N sodium hydroxide (150 mL), and extracted with diethyl ether (2×150 mL). The combined ether extracts were washed with water, dried (MgSO4), filtered and concentrated by rotary evaporation. The residue was purified by flash chromatography on silica gel, using 5% ethyl acetate: hexane as eluent, to yield 1.4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com