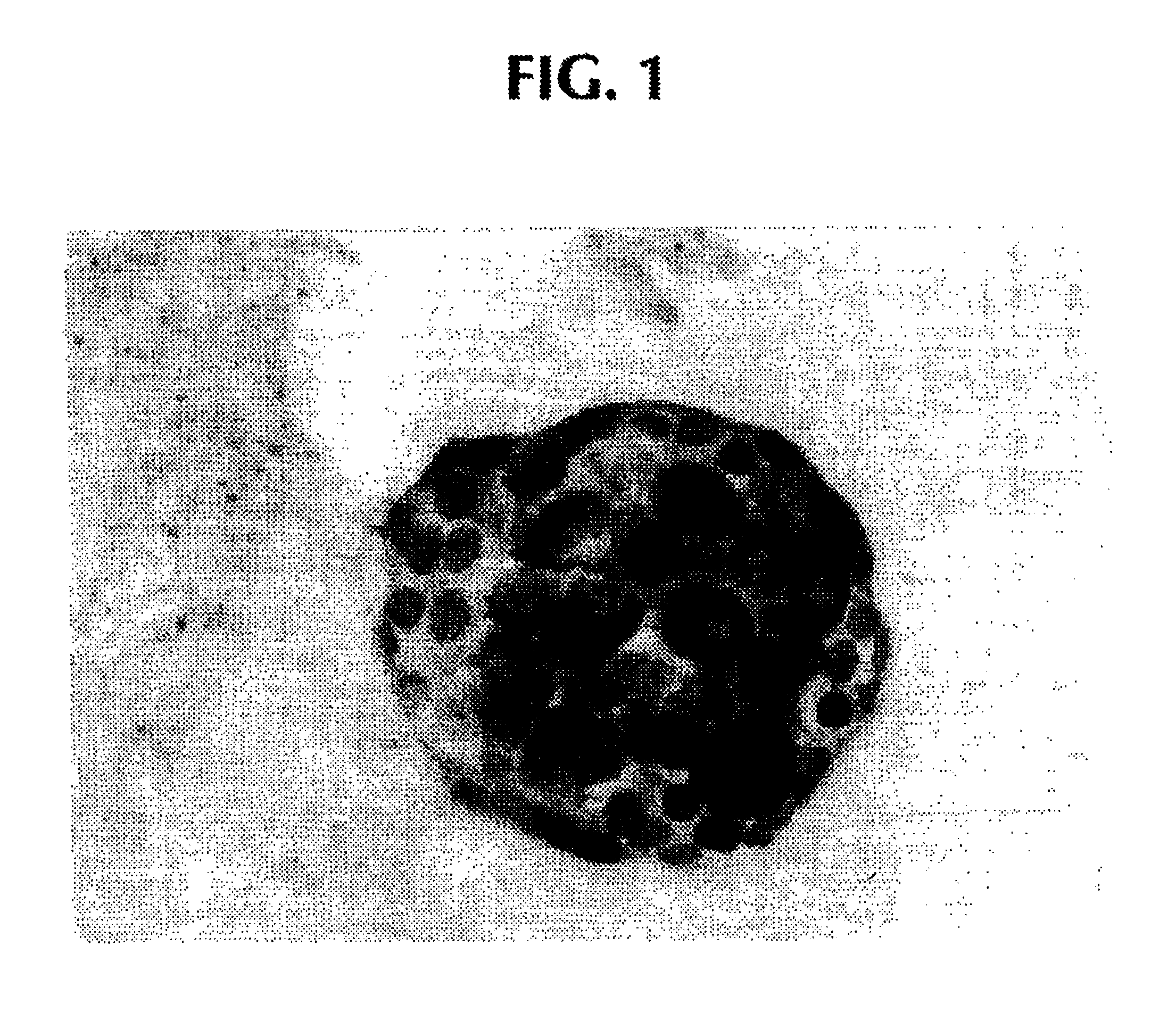
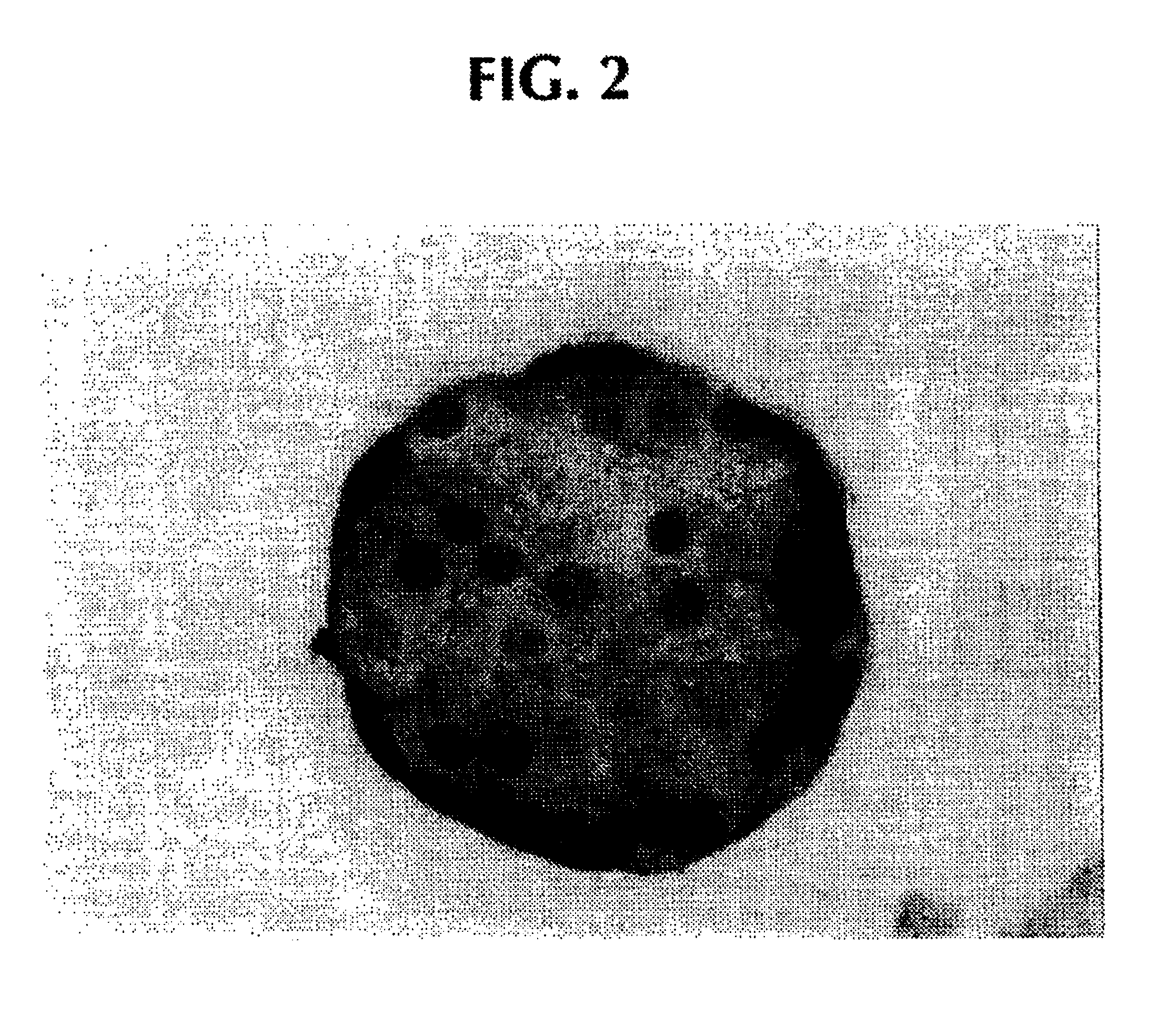
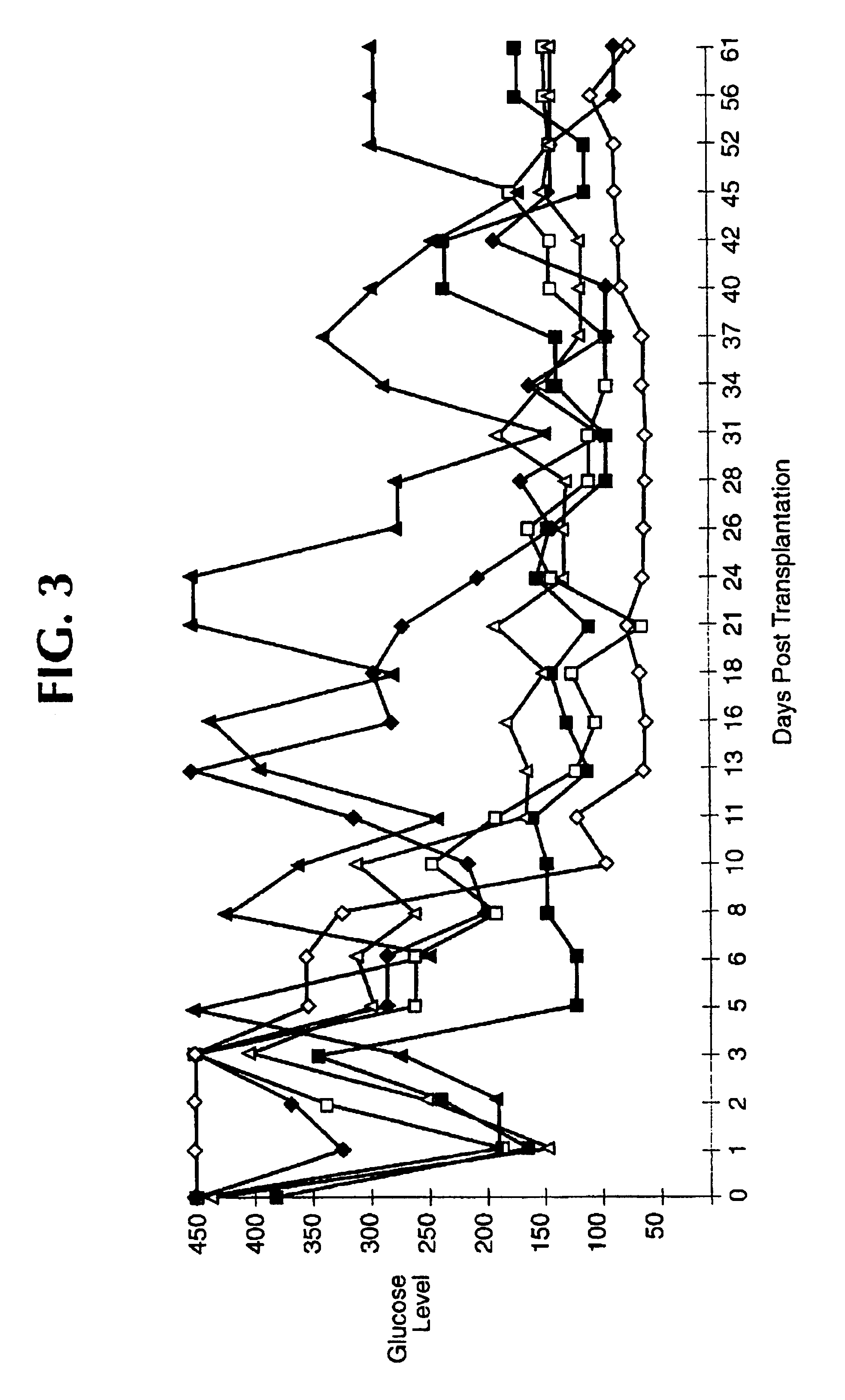
Preparation of agarose coated, solid agarose beads containing secretory cells
a technology of agarose and agarose beads, which is applied in the field of macroencapsulation of secretory cells, can solve the problems of limited clinical application of pancreatic islet transplantation, and inability to achieve effective long-term methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0044]Example I
[0045]Pancreatic Islet Isolation
[0046]Pancreatic islets were isolated from rats by a modification of the method disclosed in Gotbh et al., Transplantation 40:437(1985).
[0047]Collagenase solution (collagenase Type XI, Sigma Chemical, St. Louis, Mo.; 1 mg / ml containing 2 mg / ml of Sigma, Type V, bovine serum albumin and 1 mg / ml CaCl2) was injected into the pancreas via the common bile duct. (Gotoh et al. Transplantation 40:437 (1985), Supra). The pancreas was removed and collected in a flask maintained on ice. Once pancreata from 4 rats had been collected, the flask was placed in a waterbath, at 38° C., for 30 minutes. The resulting digested tissue was washed 4 times in cold (8° C.) HBSS (Hank's Balanced Salts Solution).
[0048]Undigested tissue, large lymph nodes, and other extraneous material were removed by repeated mobilization of the tissue, followed by removal of the supernatant. Purified, islets were isolated on a discontinuous Ficoll gradient, consisting of 25%, 23...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com