Nonpeptide insulin receptor agonists
a technology of insulin receptor and agonist, which is applied in the direction of instruments, peptide/protein ingredients, biocide, etc., can solve the problems of unresolved complete mechanism, non-peptide substances which can exert the effect of insulin on the receptor, and the inability to administer the hormone by injection, so as to achieve the effect of reducing blood glucos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Apparent Effect of TER12 on Insulin Receptor Kinase Autophosphorylation
A.
[0059]This assay is a modified form of that described in Hagino, H. et al. Diabetes (1994) 43:274-280. Briefly, human insulin receptors (hIR) were partially purified from placental extracts or from cell line IM-9. The partially purified hIRs were captured into microplate wells by incubating them for 90 minutes with wells coated with a monoclonal antibody to hlR. The wells were then treated with various dose levels of insulin and / or test compounds for 15 minutes at room temperature; ATP (10 μM) was then added to permit kinase activity to proceed. After 60 minutes, the wells were washed, and then treated for 60 minutes with biotinylated antibody directed against phospho tyrosine (PY-20) and unbound materials again washed away. The wells were then incubated with a conventional streptavidin peroxidase system for 30 minutes to assess the level of phosphorylated tyrosine.
[0060]When tested in this assay, insulin gave ...
example 2
Additional Compounds with TER12-Like Activity
[0067]Using substructure searching based on the TER12 molecule, 42 candidate compounds were obtained and assayed according to the procedure of Example 1.
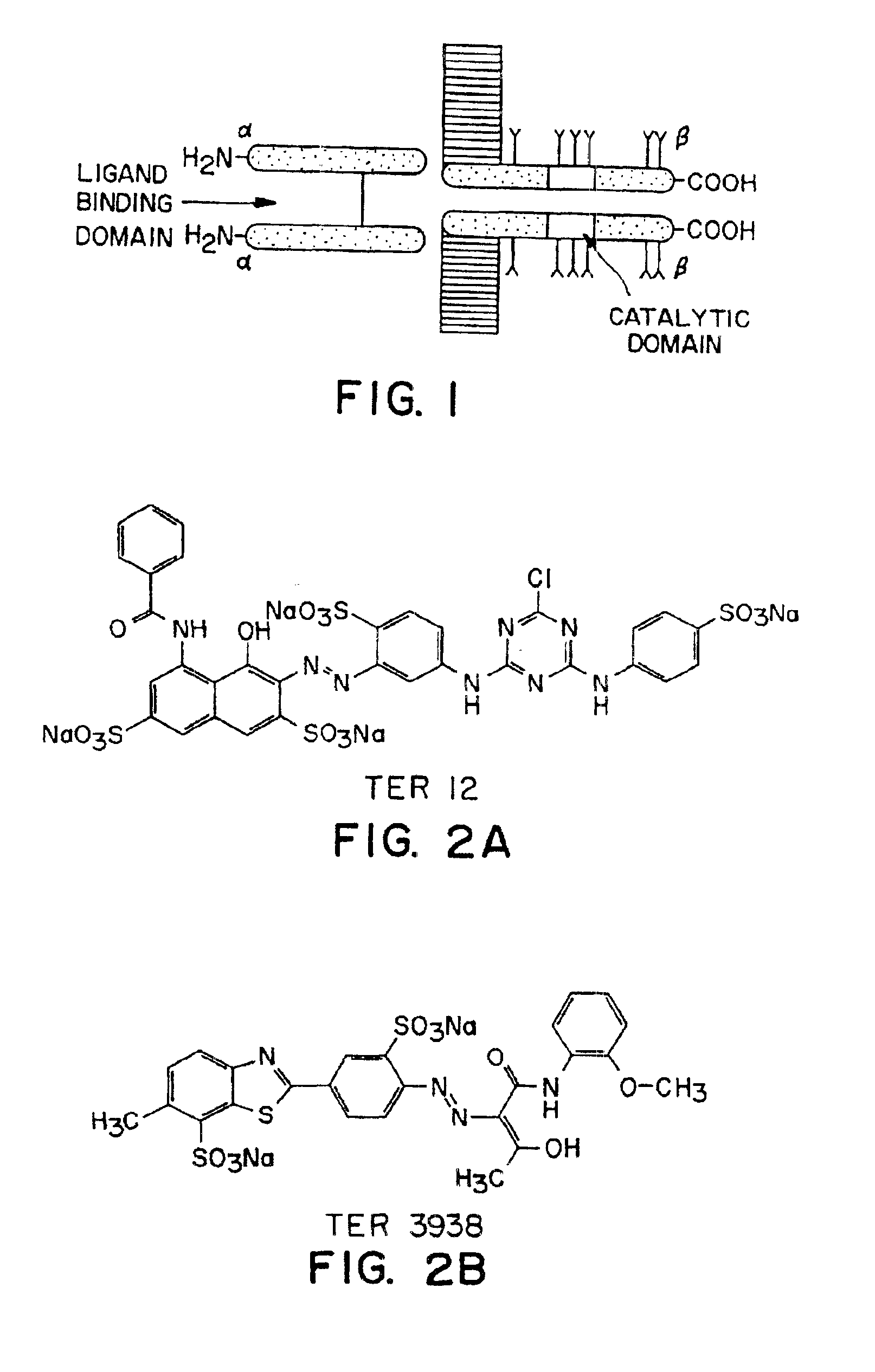
[0068]A sample containing TER3938, shown in FIG. 2B, also showed agonist activity. TER3938, shown in FIG. 2B and known as Direct Yellow No. 27, showed an EC50 of 8 μM in this in vitro assay; it also enhanced the activity of insulin in stimulating autophosphorylation of insulin receptor on intact IM-9 cells. In addition, a sample containing TER3935, shown in FIG. 2C, was active in the IR kinase assay.
example 3
Identification of an Active Component of TER12 and TER3938-Containing Samples
[0069]TER12 was synthesized by the reaction scheme shown in FIG. 3. TER12 synthesized using this scheme, and TER12 when extensively purified from commercial sources were active in the assays set forth in Example 1.
[0070]In addition, the sample containing TER3938, also obtained from commercial sources, when purified to 95% purity by reverse-phase HPLC, retained its activity; however, when this sample was washed with aqueous sodium carbonate, the insoluble compound shown in FIG. 2B as TER3938 was less active in the IR kinase assay; the aqueous layer, however, retained full activity. These results led to the conclusion that some of the activity shown in samples purportedly containing only TER12 and TER3938 was due to a minor component. This minor component was postulated to be Component A, which has the formula shown in FIG. 2F. Component A, obtained from commercial sources, was purified by C-18 reverse-phase ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com