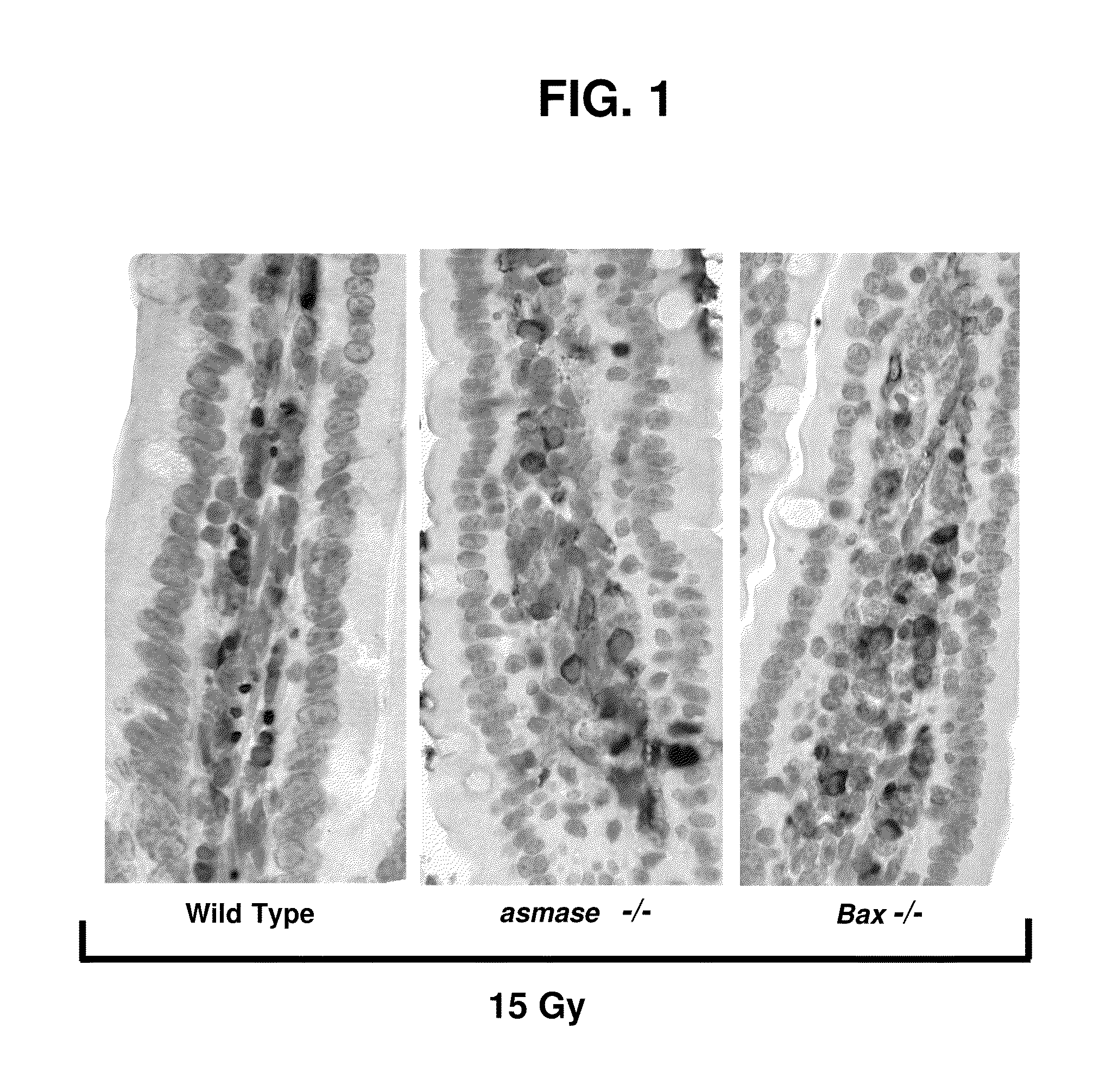
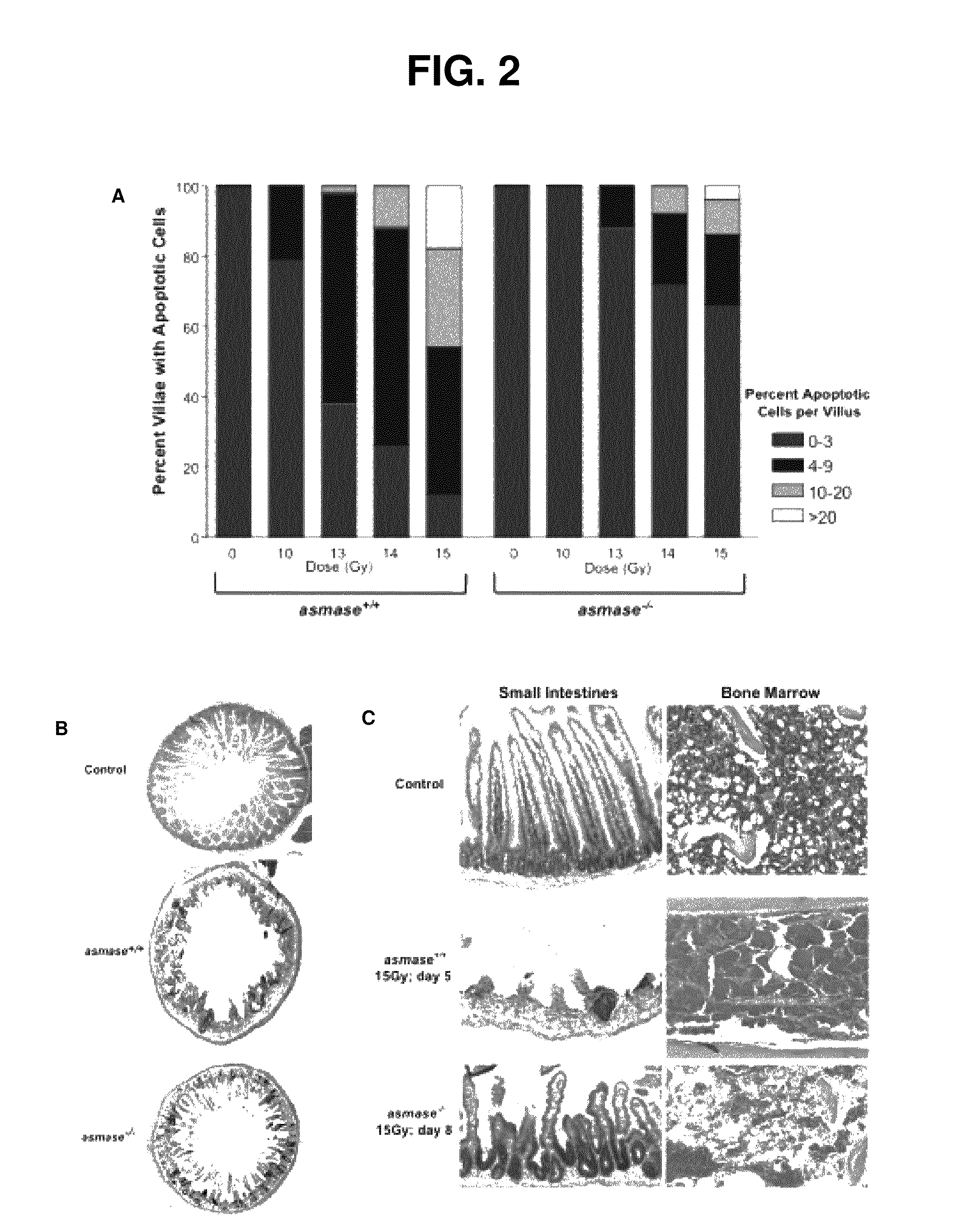
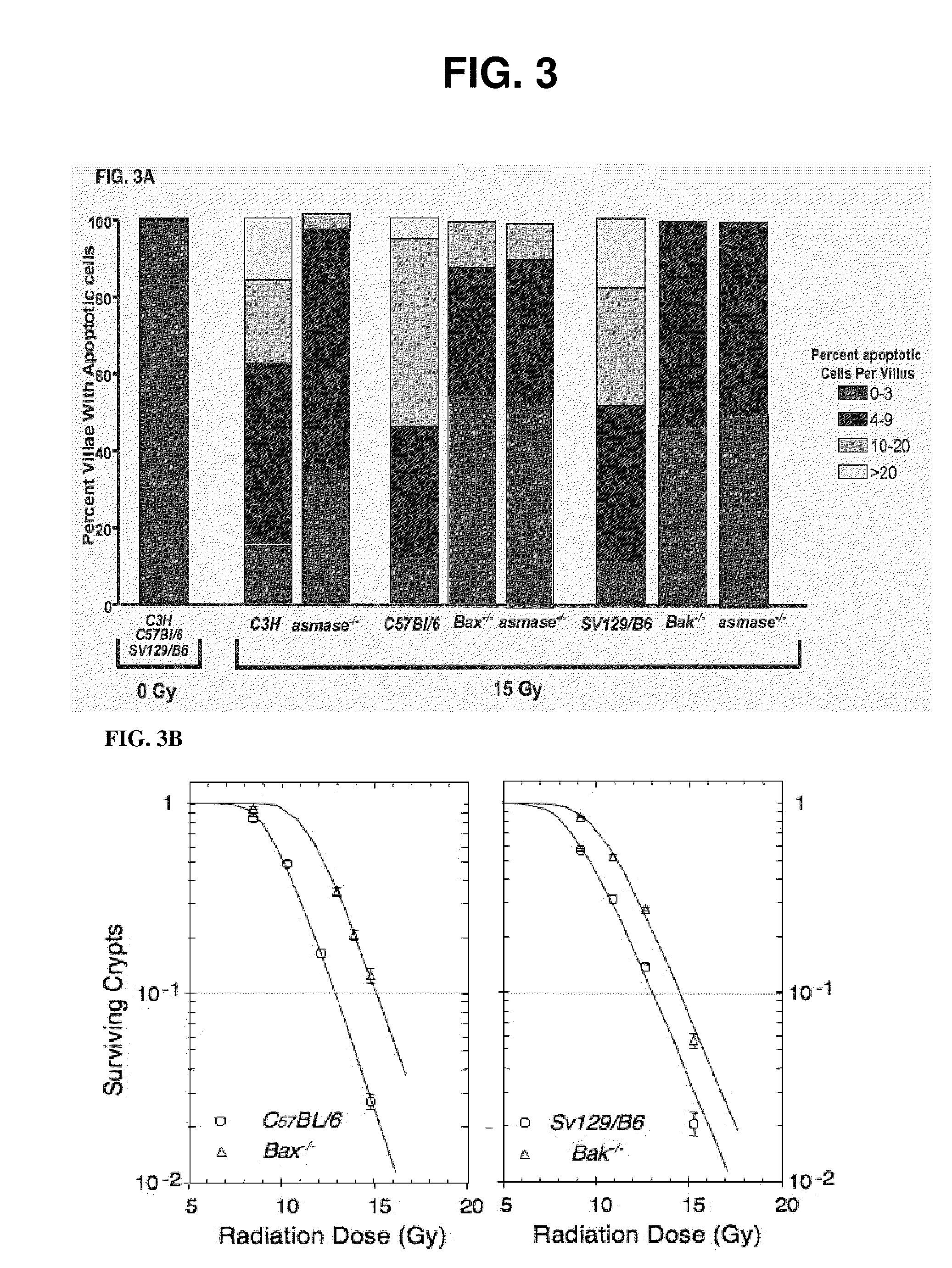
Methods for treating GI syndrome and graft versus host disease
a technology of graft and host disease, which is applied in the field of methods for treating and preventing gi syndrome and graft versus host disease, can solve the problems of increasing the incidence of gvhd, limiting the effective use of this therapy for cancer treatment, and patients susceptible to neoplastic relapse or infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials & Methods
Cell Culture and Stimulation
[0155]Wild-type (clone E6-1), caspase 8− / − (clone 19.2) and FADD− / − (clone 12.1) Jurkat T lymphocytes were obtained from ATCC (Rockville, Md.). Cells were grown in a 5% CO2 incubator at 37° C. in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 10 mM Hepes (pH 7.4), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 100 units / ml penicillin, and 100 μg / ml streptomycin. Prior to stimulation with UV-C or anti-Fas, cells were resuspended in fresh medium and allowed to acclimate for 4 hours. Jurkat cells were then treated with 50 ng / ml anti-FasCH-11 activating antibody (Upstate Biotechnology, Lake Placid N.Y.) or 50 Joules / m2 UV-C using an FB-UVXL-1000 Crosslinker (Fisher Biotech, Pittsburgh Pa.), unless otherwise indicated. For platform studies, Jurkat cells were incubated with CH-11 at 4° C. for 20 min to insure uniform receptor engagement, and warmed to 37° C. to initiate stimulation.
[01...
example 2
Role of Asmase in GVHD
[0179]Small intestine, liver and skin were harvested from asmase+ / + and asmase− / − recipients 21 days following transplantation of BM with or without 3×106 T cells. Hematoxylin & Eosin stained liver sections revealed hepatic GvHD, characterized by lymphocyte infiltration (FIG. 22A, arrows), portal tract inflammation, endotheliitis (FIG. 22A, right panels) and loss of hepatic architecture was less prominent in asmase− / − compared to asmase+ / + recipients. Similarly, intestinal GvHD, including villus blunting, lamina propria inflammation, crypt stem cell loss and destruction and mucosal atrophy were less prominent in asmase− / − recipients (FIG. 22B, arrows indicate apoptotic cells). Semiquantitative histopathologic analyses revealed that asmase+ / + recipients of allogeneic bone marrow and T cells scored significantly higher than littermates receiving only BM in liver (Table 1, 15.7±1.5 vs. 8.3±2.7, p+ / + littermates).
[0180]GvHD-associated organ injury is associated wit...
example 3
ASMase Deficiency Protects Against Inflammation
[0183]We next conducted experiments showing that host ASMase inactivation attenuates Th1 / Th2 cytokine profile and CD8+ T cell proliferation in acute GvHD. Initial CTL-mediated tissue damage propagates a feed-forward response requiring CD4+ Th1 cytokine secretion and consequent alloreactive CD8+ clonal expansion for acute GvHD to proceed, followed by inflammatory cytokine storm. To assess the impact of ASMase on serum cytokine levels during GvHD, serum Th1 cytokines IL-2 And IFN-γ and Th2 cytokines IL-1β and TNF-α were quantified 7 and 14 days following transplantation of LP BM with or without splenic T cells. In the minor mismatched model, addition of T cells to the allograft increased IL-2 and IFN-γ from 15.7±4.1 to 30.3±2.8 and 3.7±1.0 to 109.9±18.9 pg / ml serum, respectively (p+ / + hosts) and IL-1β and TNF-α to 2.9±1.2 and 17.3±3.0 pg / ml serum, respectively, (p+ / + hosts and not significant) on day 7 (Table 3). Attenuation of serum cyto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength range | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com