Vγ9Vδ2 T cell proliferation agent, method for producing activated Vγ9Vδ2 T cells, and uses thereof
a t cell and proliferation agent technology, applied in the field of v9v2 t cell proliferation agent, can solve the problems of difficult to obtain a sufficient amount of v9v2 t cells for use in immunotherapy, inability to proliferate v9v2 t cells insufficient amounts, and causing patients much pain and burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Proliferation of Vγ9Vδ2 T Cells
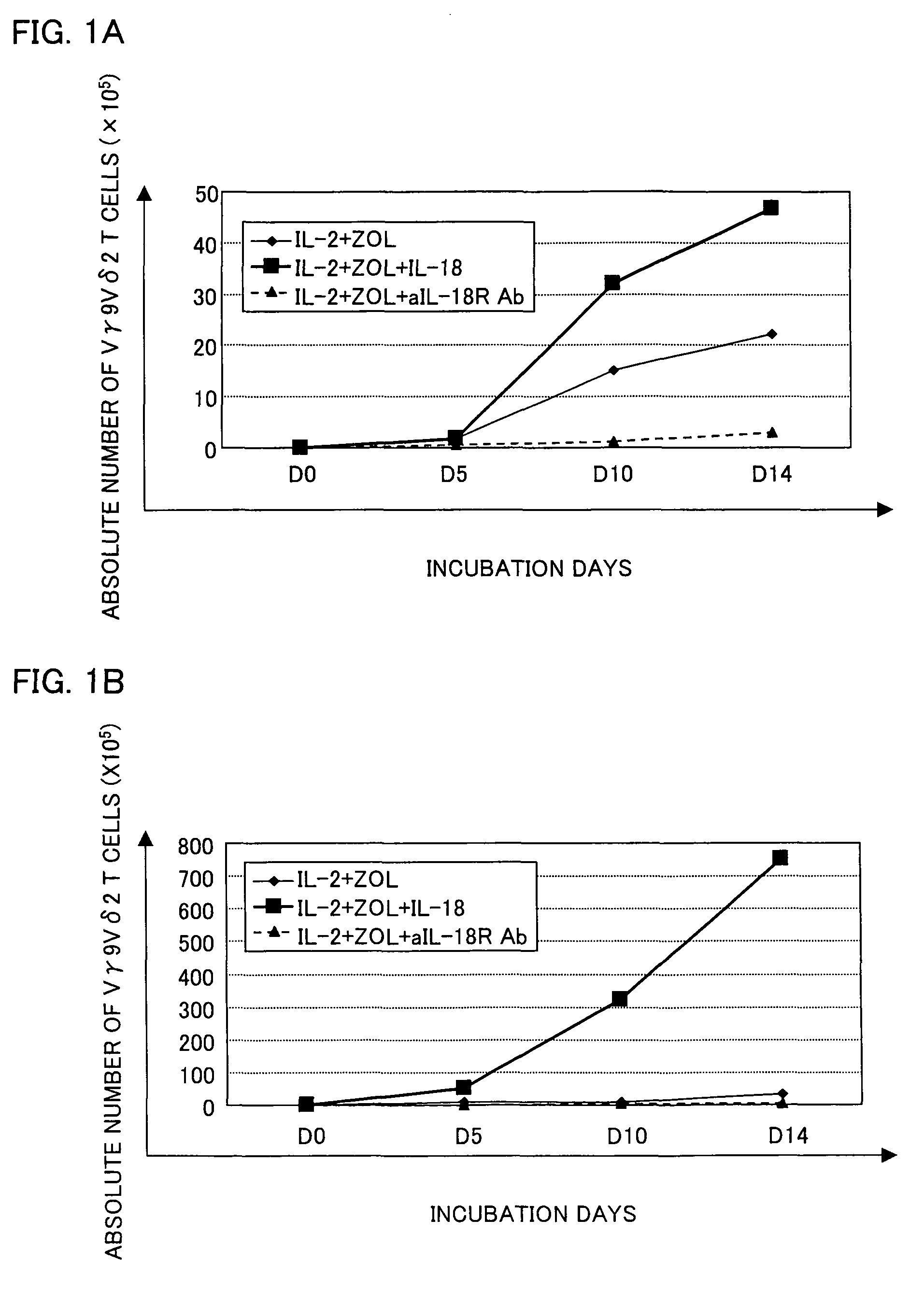
[0058]Peripheral blood was collected from a healthy adult, and PBMCs were separated from the peripheral blood by Ficoll-Hypaque density gradient centrifugation (conditions: at 2000 rpm for 20 minutes at room temperature (25° C.), using a swing rotor TS-7 (available from TOMY SEIKO Co., Ltd.)).
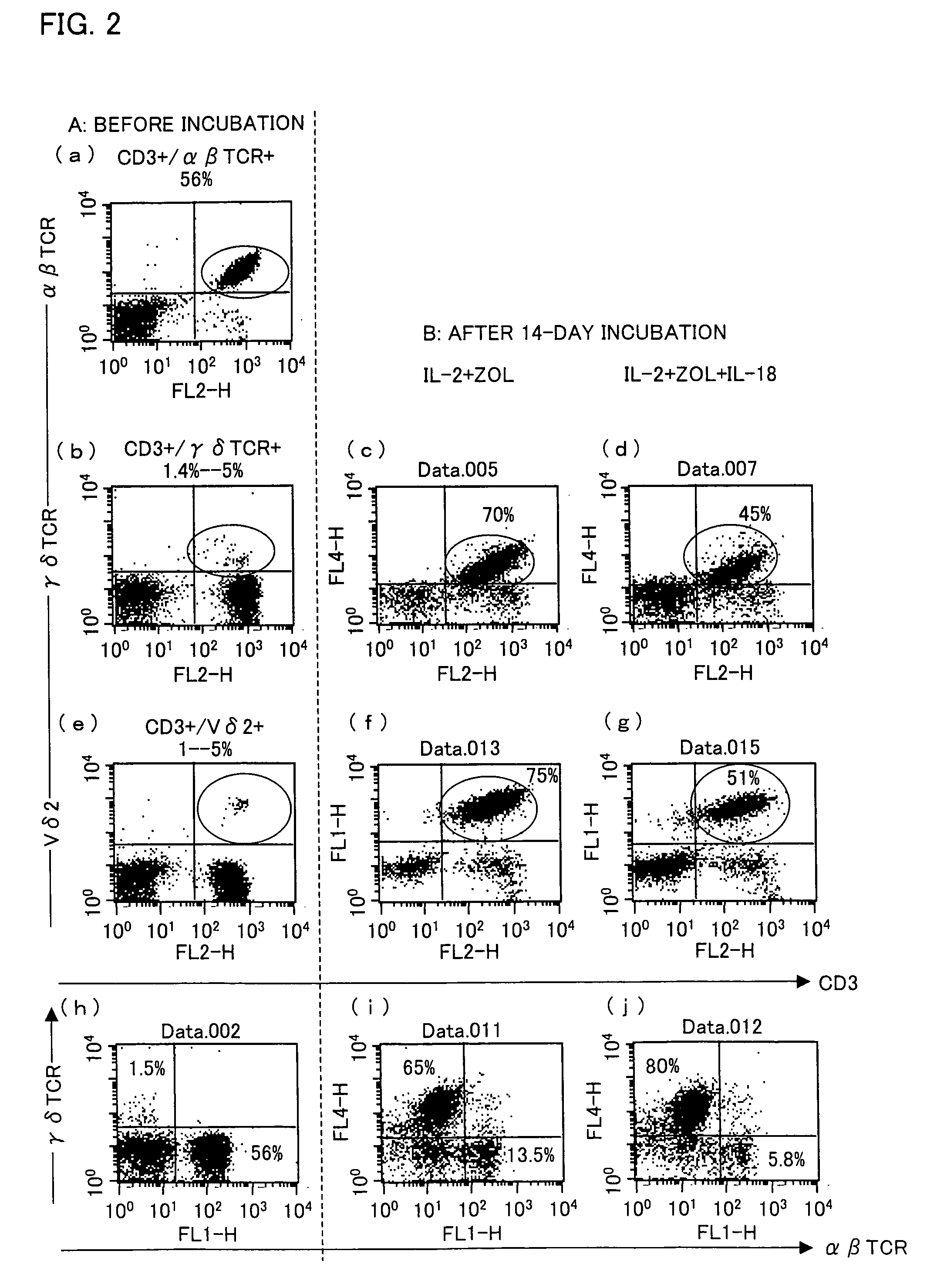
[0059]A PRMI 1640 medium containing 5% human type-AB serum was supplemented with zoledronate (with a final concentration of 1 μM), IL-2 (with a final concentration of 10 ng / ml), and IL-18 (with a final concentration of 100 ng / ml). The PBMCs were suspended in the culture solution so as to occur in a concentration of 5×105 / ml to 10×105 / ml. As shown in (e) of FIG. 2, the PBMCs contained about 1% to 5% Vγ9Vδ2 T cells.
[0060]The PBMCs were cultured in a CO2 incubator at 37° C. in the presence of 5% CO2 for 14 days during which a fresh medium was added therein every 3 to 5 days. The fresh medium was prepared by adding zoledronate, IL-2, and IL-18 to the fresh medium so a...
example 2
Physiological Action of Activated Vγ9Vδ2 T Cells
example 2-1
Expression of Surface Antigen NKG2D
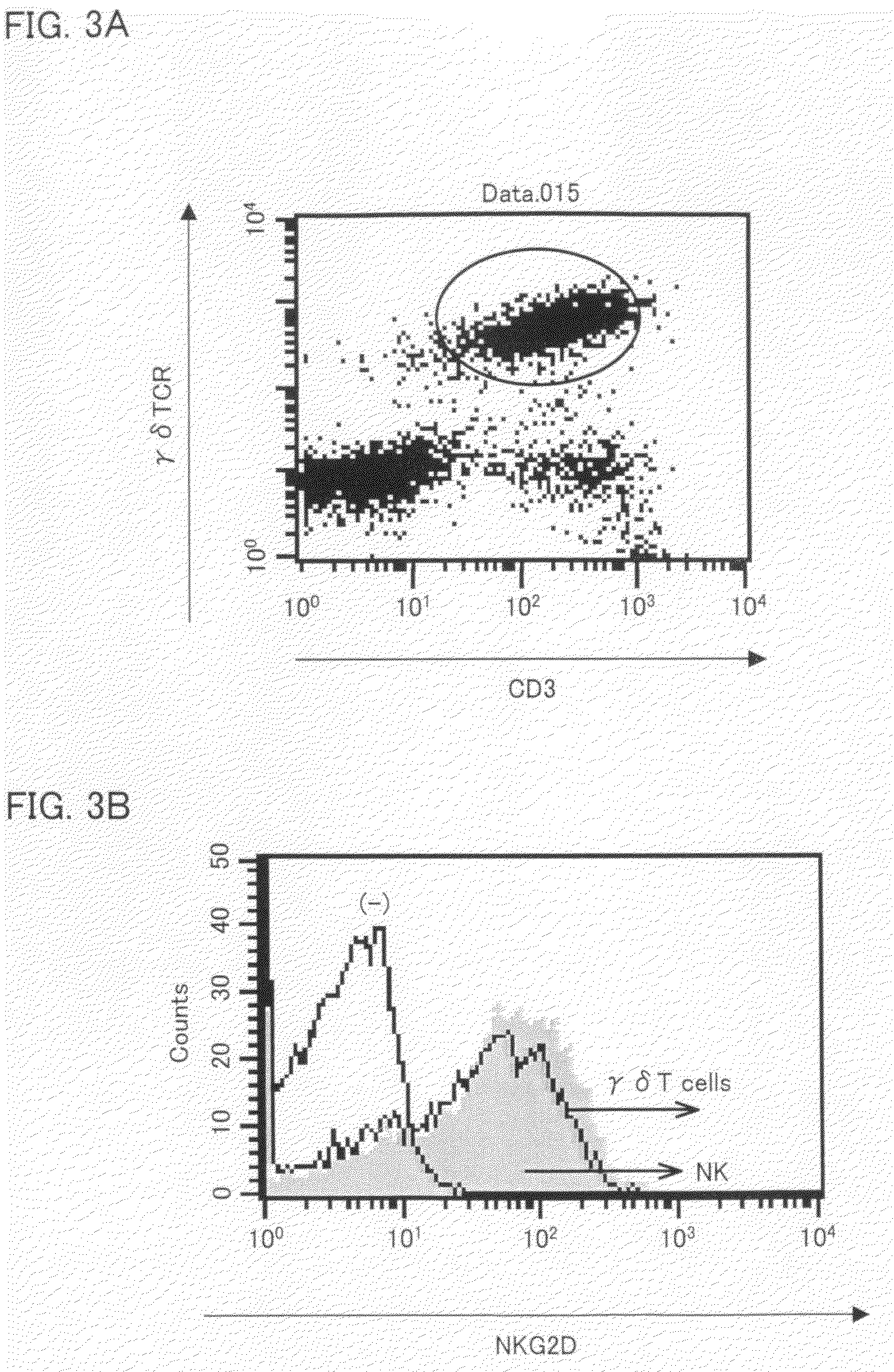
[0076]Flow-cytometric analysis of the Vγ9Vδ2 T cells (activated Vγ9Vδ2 T cells) proliferated through stimulation by the IL-2, the zoledronate, and the IL-18 as described above was conducted. The flow-cytometric analysis showed that the Vγ9Vδ2 T cells strongly expressed NKG2D, which plays a critical role in antitumor action. This suggests that the activated Vγ9Vδ2 T cells had a strong antitumor action. FIG. 3A shows the expression of NKG2D on the activated Vγ9Vδ2 T cells. FIG. 3A is identical to FIG. 2(g). The cells within the circle are Vγ9Vδ2 T cells. It is clear from FIG. 3B that the Vδ2 T cells stimulated by the IL-2, the zoledronate, and the IL-18 strongly expressed NKG2D.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com