Methods for producing acetic acid
a technology of acetic acid and acetic acid, which is applied in the field of methods for producing acetic acid, can solve the problems of reducing the quality of produced acetic acid, requiring a great deal of energy to remove water, and the extremely low hydrogen partial pressure generally fails to ensure a sufficiently high catalytic activity, so as to reduce the formation of by-products, reduce the reaction rate of acetic acid, and efficiently produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
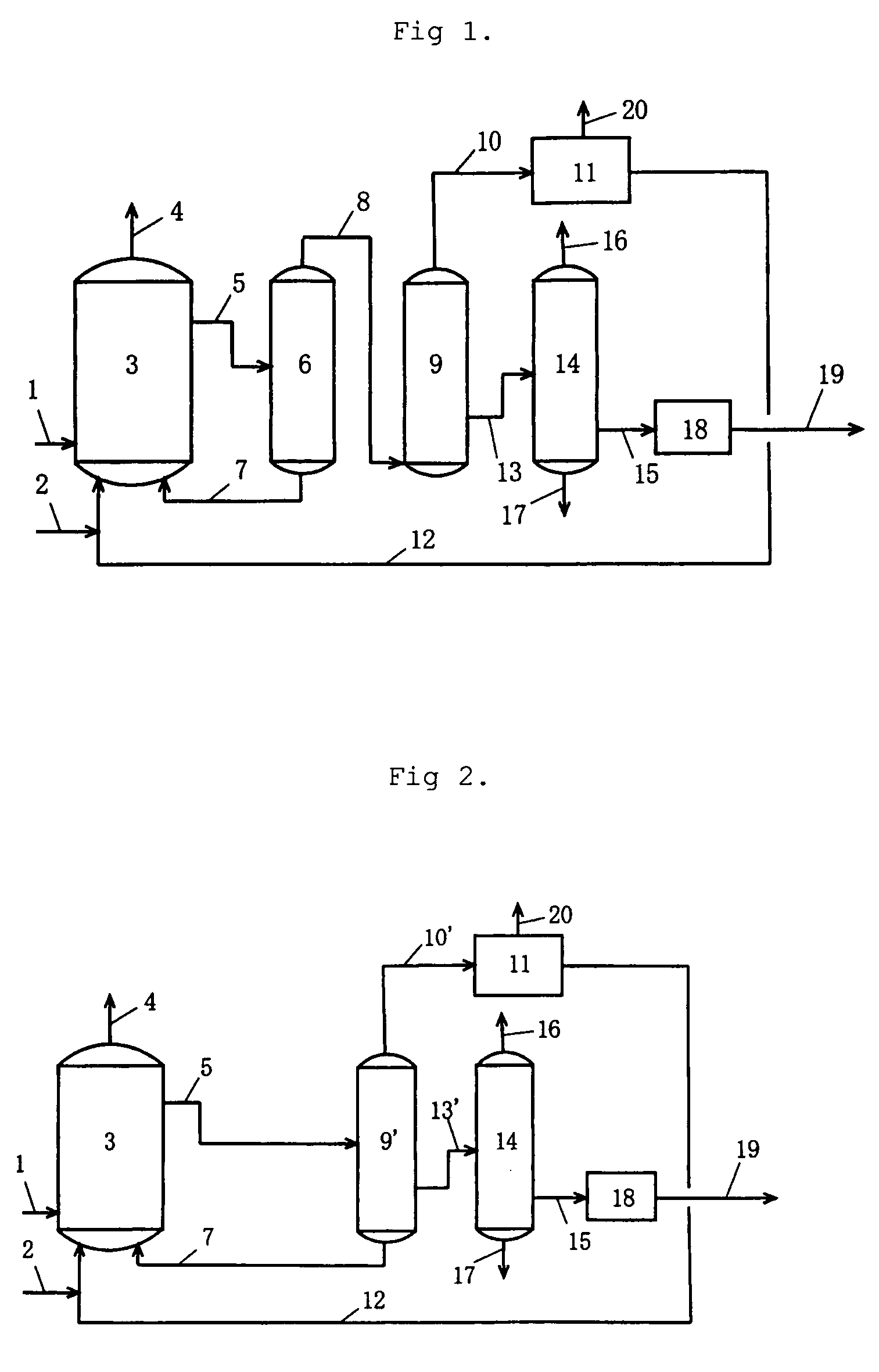
[0080]To a reactor 3 were continuously fed reaction raw materials (methanol 2 and carbon monoxide 1), a rhodium catalyst mixture 7 (containing a rhodium catalyst, an iodide salt, and acetic acid), and low-boiling components 12 (containing methyl iodide, methyl acetate, and water). The rhodium catalyst mixture 7 and the low-boiling components 12 had been recycled from a purification system. A reaction was thus conducted at a reaction pressure of 3.0 MPaG, a carbon monoxide (CO) partial pressure of 1.3 MPaA, a hydrogen (H2) partial pressure of 0.03 MPaA, a reaction temperature of 188° C., and, of the reaction mixture, a methyl acetate (MA) content of 5.5 percent by weight, a rhodium (Rh) content of 800 ppm by weight, and a lithium iodide (LiI) content of 9.6 percent by weight. The reaction mixture 5 was flashed using an evaporator 6, and a high-boiling component containing the catalytic component (rhodium catalyst mixture 7) was pressurized by a pump and recycled to the reactor 3. Fla...
example 2
[0084]To a reactor 3 were continuously fed reaction raw materials (methanol 2 and carbon monoxide 1), a rhodium catalyst mixture 7 (containing a rhodium catalyst, an iodide salt, and acetic acid), and low-boiling components 12 (containing methyl iodide, methyl acetate, and water). The rhodium catalyst mixture 7 and the low-boiling components 12 had been recycled from a purification system. A reaction was thus conducted at a reaction pressure of 2.7 MPaG, a carbon monoxide partial pressure of 1.2 MPaA, a hydrogen partial pressure of 0.031 MPaA, a reaction temperature of 186° C., and, of the reaction mixture, a methyl acetate content of 5.5 percent by weight, a rhodium content of 650 ppm by weight, and a lithium iodide content of 9.9 percent by weight. The reaction mixture 5 was flashed using an evaporator 6, and a high-boiling component containing the catalytic component (rhodium catalyst mixture 7) was pressurized by a pump and recycled to the reactor 3. Flashed components 8 were fe...
example 3
[0088]To a reactor 3 were continuously fed reaction raw materials (methanol 2 and carbon monoxide 1), a rhodium catalyst mixture 7 (containing a rhodium catalyst, an iodide salt, and acetic acid), and low-boiling components 12 (containing methyl iodide, methyl acetate, and water). The rhodium catalyst mixture 7 and the low-boiling components 12 had been recycled from a purification system. A reaction was thus conducted at a reaction pressure of 3.5 MPaG, a carbon monoxide partial pressure of 1.8 MPaA, a hydrogen partial pressure of 0.03 MPaA, a reaction temperature of 188° C., and, of the reaction mixture, a methyl acetate content of 5.3 percent by weight, a rhodium content of 800 ppm by weight, and a lithium iodide content of 10.9 percent by weight. The reaction mixture 5 was flashed using an evaporator 6, and a high-boiling component containing the catalytic component (rhodium catalyst mixture 7) was pressurized by a pump and recycled to the reactor 3. Flashed components 8 were fe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| partial pressure | aaaaa | aaaaa |
| partial pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com