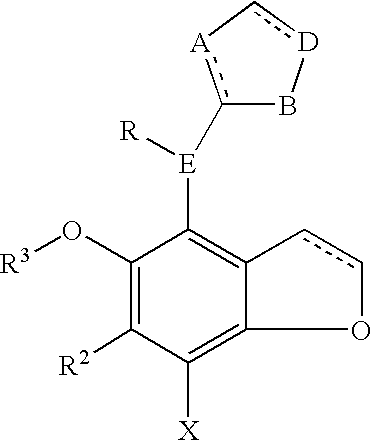
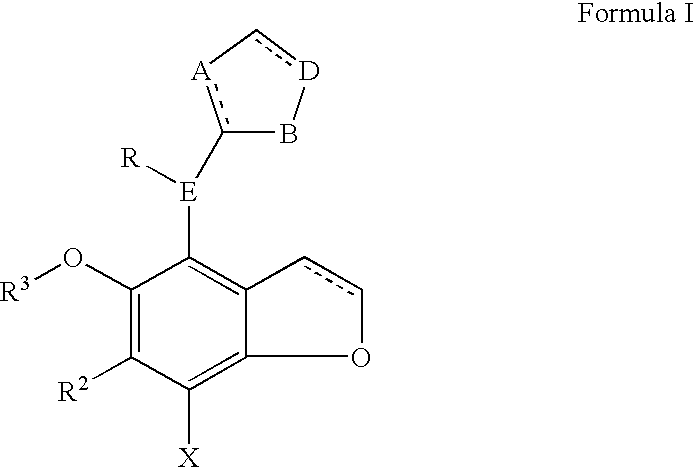
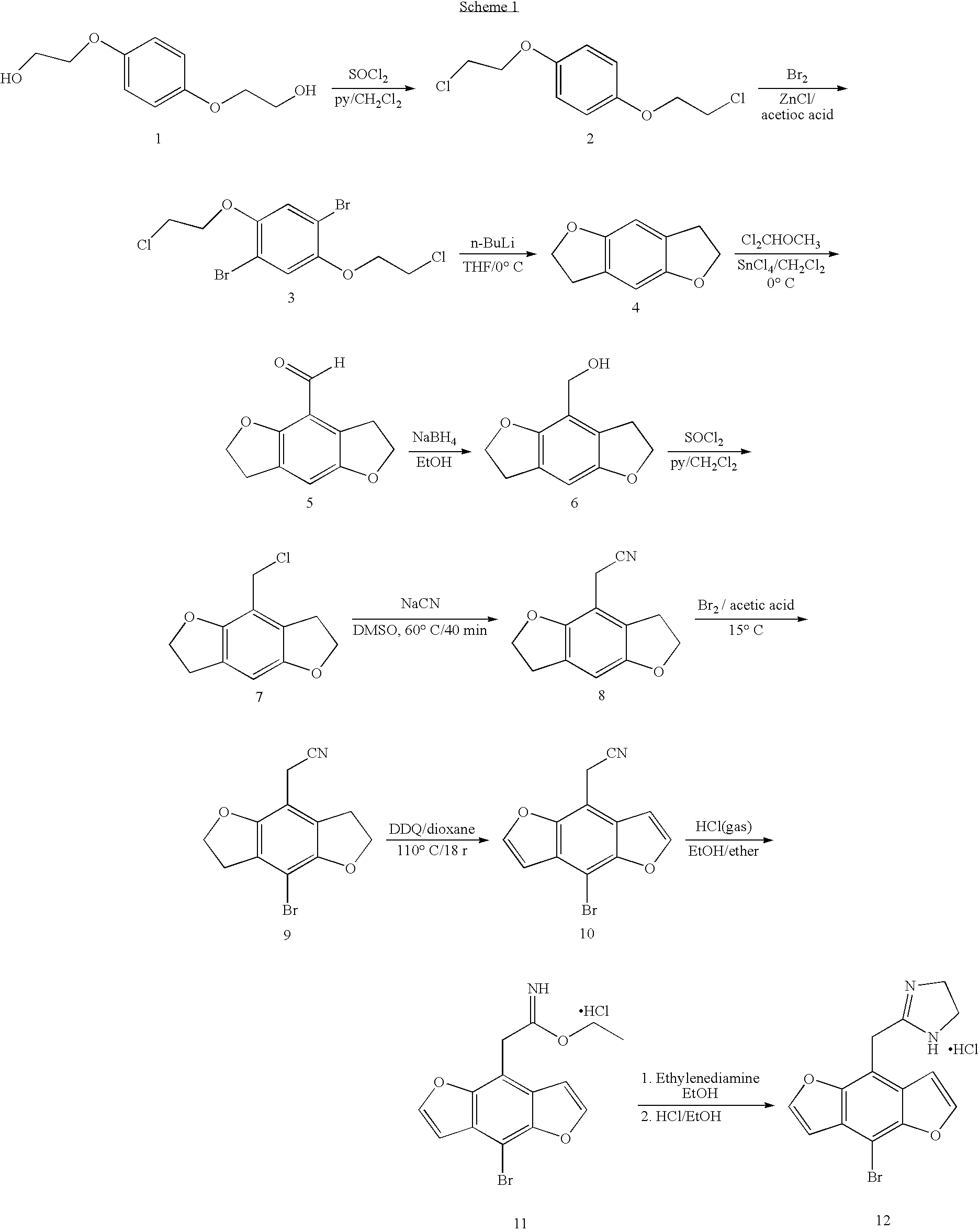Benzodifuranimidazoline and benzofuranimidazoline derivatives and their use for the treatment of glaucoma
a technology of benzofuranimidazoline and benzofuranimidazoline, which is applied in the direction of biocide, drug composition, and elcosanoid active ingredients, can solve the problems of individuals who do not respond well, considered to be at high risk for the eventual development of visual loss,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthetic Scheme for 2-(8-bromo-benzo-[1,2-b;4,5-b′]difuran-4-yl) imidazoline hydrochloride
[0031]Examples of the compounds of this invention may be prepared by the synthetic route describe by Scheme 1. Briefly, the commercially available bis ethanol ether is treated with thionyl chloride in the presence of a organic base preferably pyridine in a solvent such as methylene chloride to form 2. The halogenated ether 2 is brominated using bromine in the presence of a Lewis acid such as zinc chloride in a solvent such as acetic acid to give compound 3. The di-bromide is cyclized to 4 with n-butyl lithium in a solvent such as dioxane or tetrahydrofuran maintained at a temperature of −40 to 0° C. Formylation with dichloromethyl methyl ether in the presence of stannic chloride in an inert solvent such as methylene chloride provides 5. Reduction of the aldehyde with sodium borohyride in a solvent such as ethanol or isopropyl alcohol yields the alcohol 6. The alcohol is converted to the chlori...
example 2
2-(8-bromo-benzo-[1,2-b;4,5-b′]difuran-4-yl) imidazoline hydrochloride
[0033]2-(8-Bromo-benzo-[1,2-b;4,5-b′]difuran-4-yl) imidazoline hydrochloride was prepared by the multi-step procedure described below.
Step A: 1,4-Bis(2chloroethoxy)benzene
[0034]Bis(2-hydroxyethyl)hydroquinone (50 g, 0.25 mol) was dissolved in 500 ml of CH2Cl2 and cooled to 0° C., pyridine (48 ml, 0.6 mol) and thionyl chloride (41 ml, 0.58 ml) were added dropwise such that the temperature did not exceed 5° C. The mixture was allowed to warm to room temperature and was stirred over night. The solvent volume was reduced to 150 ml. Aqueous 2N HCl (150 ml) was added slowly and the layers were separated. The aqueous layer was extracted with CH2Cl2 (3×100 ml). The combined organic layer was washed with 2N HCl (150 ml), saturated NaCl solution (150 ml), dried over anhydrous MgSO4, filtered and evaporated to a white solid. Recrystallization from is ethanol afforded a white solid (73 g). CIMS m / z 236 (M+H)+.
Step B: 1,4-Bis(...
example 3
[0045]In order to determine the relative affinities of serotonergic compounds at the 5-HT2 receptors, their ability to compete for the binding of the agonist radioligand [125I]DOI to brain 5-HT2 receptors is determined as described below with minor modification of the literature procedure (Johnson et al. 1987). Aliquots of post mortem rat cerebral cortex homogenates (400 μl) dispersed in 50 mM TrisHCl buffer (pH 7.4) are incubated with [125I]DOI (80 pM final) in the absence or presence of methiothepin (10 μM final) to define total and non-specific binding, respectively, in a total volume of 0.5 ml. The assay mixture is incubated for 1 hour at 23° C. in polypropylene tubes and the assays terminated by rapid vacuum filtration over Whatman GF / B glass fiber filters previously soaked in 0.3% polyethyleneimine using ice-cold buffer. Test compounds (at different concentrations) are substituted for methiothepin. Filter-bound radioactivity is determined by scintil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com